Light-Enabled Reversible Hydrogen Storage of Borohydrides Activated by Photogenerated Vacancies
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Borohydrides, known for ultrahigh hydrogen density, are promising hydrogen storage materials but typically require high operating temperatures due to their strong thermodynamic stability. Here we introduce a novel light-induced destabilization mechanism for hydrogen storage reaction of borohydrides under ambient conditions via photogenerated vacancies in LiH. These vacancies thermodynamically destabilize B–H bonds through the spontaneous “strong adsorption” of BH4 groups, which trigger an asymmetric redistribution of electrons, enabling hydrogen release at near room temperature, approximately 300 °C lower than the corresponding thermal process. By utilizing specially designed “nano-photothermal reactors”, which optimize thermodynamic destabilization effect with nanoscale dispersed LiH and create space-confined “hotspots” to enhance hydrogen storage kinetics, we achieve an ultrahigh hydrogen storage capacity of 11.02 wt % H2 in LiBH4 using only light irradiation. This light-induced destabilization mechanism can also be extended to other alkali metal borohydrides, offering insights for developing solid-state hydrogen storage materials under mild conditions.
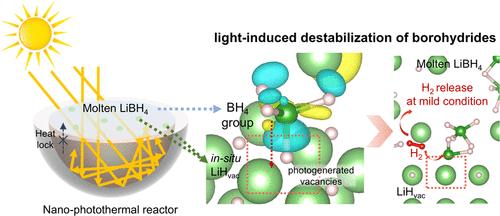
由光生空位激活的硼氢化物的光致可逆储氢
硼氢化物以超高氢密度而闻名,是一种很有前途的储氢材料,但由于其强大的热力学稳定性,通常需要较高的操作温度。本文介绍了一种新的光诱导不稳定机制,用于环境条件下硼氢化物通过LiH中的光生空位进行储氢反应。这些空位通过BH4基团的自发“强吸附”,在热力学上破坏了B-H键的稳定,引发了电子的不对称再分配,使氢在接近室温的情况下释放,比相应的热过程低约300℃。我们利用专门设计的“纳米光热反应器”,优化了纳米级分散LiH的热力学不稳定效应,并创建了空间受限的“热点”,以提高氢的储存动力学,在LiBH4中实现了11.02 wt % H2的超高储氢容量。这种光致不稳定机制也可以扩展到其他碱金属硼氢化物,为在温和条件下开发固态储氢材料提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


