Chemodivergent dearomatization of benzene-linked O-oxime esters via EnT-induced radical cross-coupling
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Radical-mediated dearomatization strategies offer a blueprint for building value-added and synthetically valuable three-dimensional skeletons from readily available aromatic starting materials. Herein, we report a novel strategy by leveraging benzene-linked O-oxime esters as triply functionalized precursors to form two distinct persistent radicals under a chemodivergent pathway. These radicals then couple with a cyclohexadienyl radical for either carboamination or carbo-aminoalkylation. Remarkably, a series of 4-(2-aminoethyl)anilines derivatives featuring all-carbon quaternary centers, along with the formation of four different types of chemical bonds, are efficiently constructed through a unique rearomatization cascade in the carboamination. Importantly, employing DMPU as the hydrogen atom transfer (HAT) donor strategically diverts the reaction pathway from the C–N bond formation towards the C–C bond formation. Our mechanistic explorations support a sequential HAT/energy transfer (EnT)/HAT cascade as the key stage for carbo-aminoalkylation involving the N-center iminyl radical. Significantly, this work demonstrates the elegant expansion of divergent C–N and C–C bond formation using the imine moiety within O-oxime esters as the bifunctional reagent, and it broadens the chemical space of both benzenes and O-oxime esters in radical-mediated transformations.
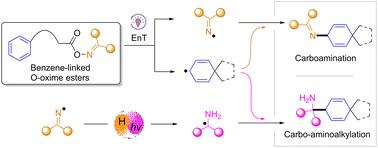
苯连接邻肟酯通过ent诱导的自由基交叉偶联的化学发散性脱芳化
自由基介导的脱芳化策略为从现成的芳香族起始材料中构建具有附加值和合成价值的三维骨架提供了蓝图。在此,我们报告了一种新的策略,利用苯连接的邻肟酯作为三功能化前体,在化学分化途径下形成两种不同的持久性自由基。然后这些自由基与环己二烯基结合进行碳胺化或碳胺烷基化。值得注意的是,一系列具有全碳季中心的4-(2-氨基乙基)苯胺衍生物,以及四种不同类型的化学键的形成,通过独特的碳胺化级联重新芳构化有效地构建。重要的是,采用DMPU作为氢原子转移(HAT)供体,战略性地将反应途径从C-N键形成转向C-C键形成。我们的机制探索支持连续的HAT/能量转移(EnT)/HAT级联是涉及n中心亚胺基自由基的碳胺烷基化的关键阶段。值得注意的是,这项工作证明了使用邻肟酯内的亚胺部分作为双功能试剂,分散的C-N和C-C键形成的优雅扩展,并拓宽了苯和邻肟酯在自由基介导转化中的化学空间。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


