Pseudo-Phosphorylated Tau Forms Paired Helical Filaments in the Presence of High-Curvature Cholesterol-Containing Lipid Membranes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
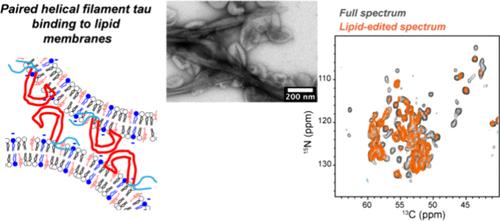
The tau protein misfolds in neurodegenerative diseases such as Alzheimer’s disease (AD). These pathological tau aggregates are associated with neuronal membranes, but molecular structural information about how disease-like tau fibrils interact with the lipid membrane is scarce. Here, we use solid-state NMR to investigate the structure of a tau construct bearing four AD-relevant phospho-mimetic mutations (4E tau) with cholesterol-containing high-curvature lipid membranes, which mimic the membrane of synaptic vesicles in neurons. We show that 4E tau adopts the AD paired helical filament (PHF) fold in the presence of the membrane at high protein concentrations. Moreover, it inserts into the membrane–water interface with an orientation that suggests possible bridging of multiple lipid vesicles. At lower protein concentrations, moderate chemical shift changes are observed, indicating a perturbation of the PHF structure by the lipids. Removal of the phospho-mimetic mutations led to a qualitatively different β-sheet conformation. These results indicate that posttranslational modifications impact the tau fibril structure more strongly than lipid membranes, but the membrane modulates the conformational equilibria of the aggregates.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


