Total Synthesis of Kasugamycin
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
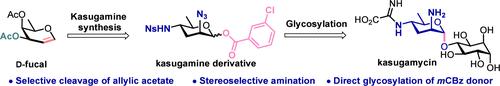
We present an efficient synthetic pathway for kasugamycin, an aminoglycoside antibiotic, utilizing naturally derived carbohydrates as starting materials. This synthesis effectively addresses stereochemical complexities by employing the selective reduction of d-fucal, which generates a crucial 3-deoxyglycal intermediate. This intermediate facilitates the introduction of amino groups at the C-2 and C-4 positions, which is essential for the synthesis of kasugamine. Subsequent glycosylation with glycosyl 1-O-m-chlorobenzoate (mCBz) donors yields a disaccharide intermediate, which is further transformed to produce kasugamycin. This streamlined approach provides a practical and effective route for the synthesis of kasugamycin and related deoxy amino sugar-containing antibiotics.
卡苏加霉素的全合成
我们提出了一种高效的氨基糖苷抗生素卡苏甘霉素的合成途径,利用天然衍生的碳水化合物作为起始材料。这种合成有效地解决了立体化学的复杂性,采用选择性还原d-fucal,这产生了一个关键的3-脱氧糖的中间体。该中间体促进在C-2和C-4位置上引入氨基,这是合成香豆精所必需的。随后与糖基1- o -m-氯苯甲酸酯(mCBz)供体糖基化产生二糖中间体,该中间体进一步转化为卡苏霉素。该方法为卡苏霉素及相关脱氧氨基糖类抗生素的合成提供了一条实用有效的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


