Intramolecular Cascade Cyclization of Cyclobutanone: Asymmetric Construction of Cyclobutanone Fused Oxa-Spirocycles
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The successful implementation of a cascade reaction involving a cyclobutyl unit has posed a significant challenge in achieving ring-retentive functionalization because of the ring’s sacrificial tendency. Herein, we have accomplished a cinchona-derived squaramide-catalyzed cascade reaction sequence, encompassing the desymmetrization of cyclobutanone, followed by an aldol reaction and, subsequently, a 1,4-addition step. This overall process offers a viable strategy to access architecturally fascinating oxa-spirocycles fused with cyclobutanone motifs in good yields with high optical purity.
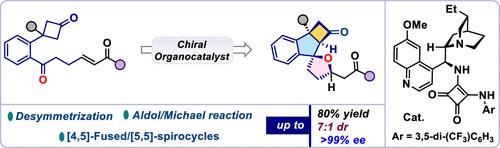
环丁酮分子内级联环化:环丁酮融合oxa -螺环的不对称结构
由于环的牺牲倾向,环丁基级联反应的成功实现对环保留功能化提出了重大挑战。在这里,我们完成了一个金鸡纳衍生的方酰胺催化的级联反应序列,包括环丁酮的去对称化,然后是醛醇反应,然后是1,4加成步骤。这个整体过程提供了一个可行的策略,以获得建筑上迷人的oxa-螺环与环丁酮基序融合,收率高,光学纯度高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


