Van der Waals Effect-Driven Charge–Transfer Excitation in 2D Polymeric Carbon Nitrides: A Vertical Excitation Model
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Atomically thin, graphene-like two-dimensional (2D) materials have been tailored to exhibit new physical and chemical properties. For g-C3N4, a typical 2D semiconductor material, the study of its interlayer coupling effect is still in its early stages, especially in the context of light excitation processes. There are limited studies of excited states. The 2D structures of the monolayer and bilayer of g-C3N4 were studied using density functional theory (DFT) and time-dependent density functional theory (TDDFT). Compared with the planar structure, the ripples in the monolayer increase the HOMO–LUMO (H–L) gap due to decreased conjugated coupling but also affect the distribution of electron–hole pairs in excited states, ultimately leading to a red shift in the light absorption peak. It was found that van der Waals (vdW) interlayer coupling affects the distribution of electron–hole pairs in the excited states of the bilayer and promotes charge–transfer excitations. The electron excitations in these excited states of the bilayer structures presented a strong and weak interval distribution between different melon chains, making it easier to form interlayer excitons or in-plane excitons. However, van der Waals forces have little effect on the excitation energy. The S0 → S1 transitions of all four structures are n → π* transitions, with the bilayer structures displaying very low oscillator strengths (<0.0006), indicating a transition barrier for the n → π* transition in the bilayer structures, which makes it challenging to measure using optical instruments in experiments.
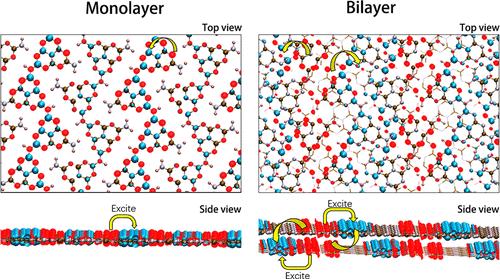
二维聚合物氮化碳中范德华效应驱动的电荷转移激发:一个垂直激发模型
原子级薄的、类石墨烯的二维(2D)材料已经被定制,以展示新的物理和化学特性。g-C3N4是一种典型的二维半导体材料,其层间耦合效应的研究还处于早期阶段,特别是在光激发过程中。关于激发态的研究有限。采用密度泛函理论(DFT)和时变密度泛函理论(TDDFT)研究了g-C3N4单层和双层的二维结构。与平面结构相比,单层结构中的波纹由于共轭耦合减少而增加了HOMO-LUMO (H-L)隙,但也影响了激发态电子-空穴对的分布,最终导致光吸收峰发生红移。研究发现,范德华耦合影响了电子-空穴对在双分子层激发态的分布,促进了电荷转移激发。这些激发态的电子激发在不同瓜链之间呈强弱间隔分布,更容易形成层间激子或面内激子。而范德华力对激发能的影响不大。这四种结构的S0→S1跃迁都是n→π*跃迁,而双层结构的振子强度非常低(<0.0006),表明双层结构中存在n→π*跃迁的跃迁势垒,这给实验中使用光学仪器进行测量带来了挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


