Understanding the Role of Potential and Cation Effect on Electrocatalytic CO2 Reduction in All-Alkynyl-Protected Ag15 Nanoclusters
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Atomically precise metal nanoclusters (NCs) have emerged as an intriguing class of model catalysts for electrochemical CO2 reduction reactions (CO2RR). However, the interplay between the interface environment (e.g., potential, cation concentration) and electron–proton transfer (ET/PT) kinetics─particularly in alkynyl-protected metal NCs─remains poorly understood. Here, we combined first-principles simulations and electrochemical experiments to investigate the role of potential and cation effect on CO2RR performance in a prototype all-alkynyl-protected Ag15(C≡C–CH3)+ cluster. Our simulations revealed that the applied reduction potential triggers the elimination of the alkynyl ligand via sequentially breaking two π-type Ag–C bonds and one σ-type Ag–C bond to expose the catalytically active Ag sites, and the barrier of the Ag–C breakage monotonically decreases with the lowering in potential. Furthermore, we show that introducing the inner-sphere Na+ ions greatly enhances *CO2 activation and promotes proton transfer to generate *COOH and *CO by forming the Na+–CO2(*COOH) complexes, while the competitive hydrogen evolution reaction (HER) from water dissociation is greatly suppressed, thus dramatically improving the selectivity of CO2 electroreduction. The electrochemical measurements further validated our predictions, where the CO Faradaic efficiency (FECO) and current density (jCO) show a pronounced dependence on the Na+ concentration. At an optimal concentration of 0.1 M NaCl, FECO can reach up to ∼96%, demonstrating the crucial role of cations in promoting the CO2RR. Our findings provide vital insights into the atomic-level reaction mechanism of the CO2RR on alkynyl-protected Ag15 NCs and highlight the important role of potential and electrolyte cation in governing the electron/proton transfer kinetics.
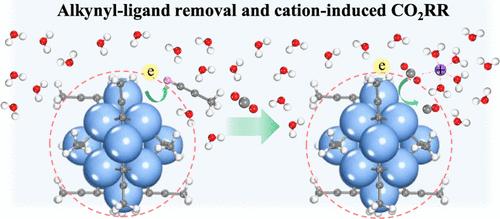
电位和阳离子效应在全炔基保护Ag15纳米团簇电催化CO2还原中的作用
原子精确的金属纳米团簇(NCs)已成为电化学CO2还原反应(CO2RR)的一类有趣的模型催化剂。然而,界面环境(如电位、阳离子浓度)和电子-质子转移(ET/PT)动力学之间的相互作用,特别是在烷基基保护的金属nc中,仍然知之甚少。在这里,我们结合第一性原理模拟和电化学实验来研究电位和阳离子效应对全炔基保护的Ag15(C≡C - ch3)+原型簇中CO2RR性能的作用。模拟结果表明,施加还原电位通过依次破坏两个π型Ag - c键和一个σ型Ag - c键来触发炔基配体的消除,从而暴露出具有催化活性的Ag位点,并且Ag - c的破坏势垒随着电位的降低而单调降低。此外,我们发现在球内引入Na+离子大大提高了*CO2的活性,并通过形成Na+ -CO2 (*COOH)配合物促进质子转移生成*COOH和*CO,同时大大抑制了水解离的竞争性析氢反应(HER),从而显著提高了CO2电还原的选择性。电化学测量进一步验证了我们的预测,CO的法拉第效率(FECO)和电流密度(jCO)与Na+浓度有明显的依赖性。在0.1 M NaCl的最佳浓度下,FECO可达~ 96%,证明阳离子在促进CO2RR中的关键作用。我们的研究结果为CO2RR在烷基基保护的Ag15 NCs上的原子水平反应机制提供了重要的见解,并强调了电位和电解质阳离子在控制电子/质子转移动力学中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


