Spectroscopy and Bonding Analysis of ArnBO+ (n = 1–3) Cations That Possess Argon–Boron Multiple Bonds
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
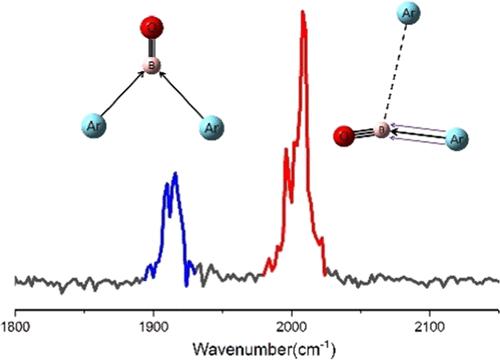
ArnBO+ (n = 1–3) complexes have been prepared and subjected to spectroscopic characterization in the gas phase. Mass-selected infrared photodissociation spectroscopy, in combination with theoretical calculations, reveals the coexistence of two nearly isoenergetic structural isomers in Ar2BO+. One isomer entails two equivalent Ar atoms chemically bound to BO+, while the other features an ArBO+ core ion accompanied by a weakly tagging argon atom. However, only the structure with an ArBO+ core ion was observed for the Ar3BO+ complex. Quantum chemical calculations using density functional theory and ab initio methods complement the experimental work. The calculations help to identify the spectroscopically observed cations whose equilibrium structures and bond dissociation energies are given. The electronic structure and bonding situation are analyzed with a variety of theoretical methods. The ArBO+ core ion is characterized as having an exceptionally strong Ar–B covalent bond with some multiple bonding character.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


