Steroids and steroid-like compounds alter the ion permeability of phospholipid bilayers via distinct interactions with lipids and interfacial water
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
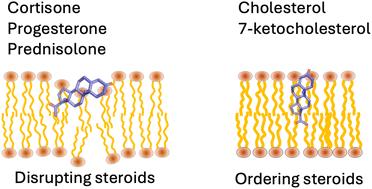
Steroids are organic compounds found in all forms of biological life. Besides their structural roles in cell membranes, steroids act as signalling molecules in various physiological processes and are used to treat inflammatory conditions. It has been hypothesised that in addition to their well-characterised genomic and non-genomic pathways, steroids exert their biological or pharmacological activities via an indirect, nonreceptor-mediated membrane mechanism caused by steroid-induced changes to the physicochemical properties of cell membranes. While the effect of cholesterol on phospholipid bilayer properties has been extensively studied, much less is known about the effect of other steroids and steroid-like molecules. Here, we combine electrical impedance spectroscopy (EIS) experiments with molecular dynamics (MD) simulations to study the effect of the steroids cortisone, prednisolone and progesterone and the steroid-like compounds enoxolone and carbenoxolone on the ion permeability and structure of phospholipid bilayers composed of the zwitterionic lipid POPC. The EIS data shows that all five compounds increase permeability, while the simulations suggest that this is accompanied by a thinning of the bilayer and reduced lipid order. We show that for steroids, a previously proposed structure–activity relationship that classifies steroids into order-promoting or order-disrupting compounds based on domain formations translates to ion permeability. We confirmed this by additional experiments with cholesterol and 7-ketocholesterol. In contrast, the previously reported relationship between log P and molecular area and a steroid being a promoter does not translate to the steroid-like compounds enoxolone and carbenoxolone. We propose that their membrane-disruption activity can be explained by their hydrogen-bonding capacity that dictates the compound's orientation at the water–lipid interface. Specifically, their membrane-disrupting ability is a result of the steroids to intercalate between lipids and form stable interactions with lipid headgroups and interfacial water, thereby pushing lipids apart and lowering the energy required for ion-induced pores, an effect previously reported for other membrane-altering small molecules.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


