Enhancing the Electrocatalytic Oxidation of 5-Hydroxymethylfurfural (HMF) via Metallic Cobalt in Au–Co Nanoparticles
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
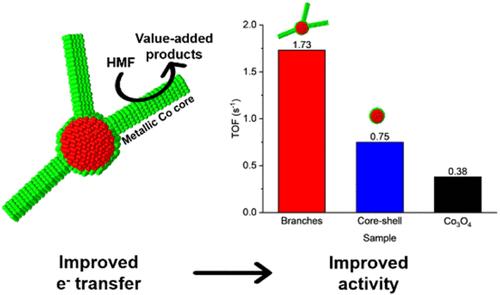
The electrochemical oxidation of 5-hydroxymethylfurfural (HMF), derived from biomass, offers a sustainable route to valuable chemicals like 2,5-furandicarboxylic acid, used for the production of chemicals, polymers, and biofuels. Cobalt-based catalysts, especially Co3O4, have shown promise for HMF electrooxidation, but their poor conductivity limits their applicability. To address this issue, we synthesized and compared the catalytic activity of Au–Co branched and core–shell nanoparticles. We demonstrate that the branched Au–Co nanoparticles exhibit significantly higher electrocatalytic activity for HMF oxidation compared with the core–shell structure and commercial Co3O4. The enhanced performance of the branched structure arises from the high surface area and preservation of a metallic cobalt core in the branches, as demonstrated by the electron microscopy analysis. Electron impedance spectroscopy measurements show that the metallic branch core results in a lower charge transfer resistance for the branched nanoparticles compared with the cobalt oxide standard. These results suggest that preserving metallic cobalt in branched structures is key for efficient charge transfer, marking a significant advancement in the understanding of the use of Co catalysts for electrochemical HMF oxidation. This work emphasizes the role of the nanoparticle morphology in enhancing catalytic activity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


