Stable Dimer Intermediates during Intercluster Reactions of Atomically Precise Nanoclusters
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
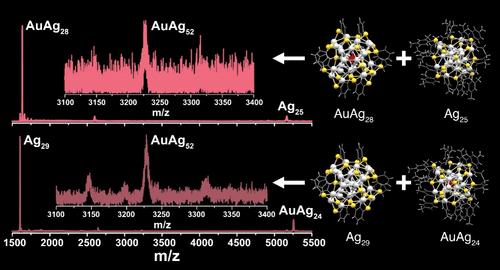
Intercluster reactions involving atomically precise noble metal nanoclusters (NCs) in solution, closely resembling reactions between molecules, are important for exploring chemistry on the nanoscale. In the present study, we conducted reactions between [Ag29(1,3-BDT)12]3– (1,3-BDT = 1,3-benzenedithiol) and center-doped [MAg24(2,4-DMBT)18]q− (q = 1 for M = Ag, Au; and q = 2 for M = Pd, Pt; 2,4-DMBT = 2,4-dimethylbenzenethiol) NCs in solution. For the first time, we report the formation of stable dimers, formed between two NCs with mixed metal–ligand interfaces. The dimeric species formed were [MAg53–xBDT12DMBT18–y]3– (x ≥ 0 and y ≥ 0), with 16 electrons in their valence shells. Here, the dimers were formed irrespective of the nature of the central atom in the NC, although the compositions were different depending on the central atom. These dimers were stable in solution for ∼2 days. The dithiol-protected [Ag29BDT12]3– part was more stable in the dimers, during fragmentation than the monothiol-protected [MAg24DMBT18]q− part. UV/vis spectroscopic and mass spectrometric analyses, along with density functional theory calculations, were used to understand the dimers. Our work highlighted the importance of the cluster interface in the stability of the dimer formed. Probing such stable dimers formed during intercluster reactions can help us understand the reaction mechanism in greater detail.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


