The hepatic clock synergizes with HIF-1α to regulate nucleotide availability during liver damage repair
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
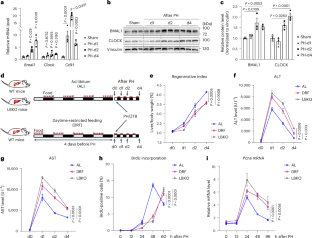
Nucleotide availability is crucial for DNA replication and repair; however, the coordinating mechanisms in vivo remain unclear. Here, we show that the circadian clock in the liver controls the activity of the pentose phosphate pathway (PPP) to support de novo nucleotide biosynthesis for DNA synthesis demands. We demonstrate that disrupting the hepatic clock by genetic manipulation or mistimed feeding impairs PPP activity in male mice, leading to nucleotide imbalance. Such defects not only elicit DNA replication stress to limit liver regeneration after resection but also allow genotoxin-induced hepatocyte senescence and STING signalling-dependent inflammation. Mechanistically, the molecular clock activator BMAL1 synergizes with hypoxia-inducible factor-1α (HIF-1α) to regulate the transcription of the PPP rate-limiting enzyme glucose-6-phosphate dehydrogenase (G6PD), which is enhanced during liver regeneration. Overexpressing G6PD restores the compromised regenerative capacity of the BMAL1- or HIF-1α-deficient liver. Moreover, boosting G6PD expression genetically or through preoperative intermittent fasting potently facilitates liver repair in normal mice. Hence, our findings highlight the physiological importance of the hepatic clock and suggest a promising pro-regenerative strategy. During liver damage repair, the circadian clock in the liver is shown to act together with HIF-1α signalling to regulate pentose phosphate pathway activity and nucleotide availability.

肝时钟与HIF-1α协同调节肝损伤修复过程中的核苷酸可用性
核苷酸的可用性对DNA的复制和修复至关重要;然而,体内的协调机制尚不清楚。在这里,我们发现肝脏中的生物钟控制戊糖磷酸途径(PPP)的活性,以支持DNA合成需求的从头核苷酸生物合成。我们证明,通过基因操作或不合时宜的喂养破坏肝脏时钟会损害雄性小鼠的PPP活性,导致核苷酸失衡。这些缺陷不仅会引起DNA复制应激,限制肝脏切除后的再生,还会导致基因毒素诱导的肝细胞衰老和STING信号依赖性炎症。机制上,分子钟激活因子BMAL1与缺氧诱导因子-1α (HIF-1α)协同调节PPP限速酶葡萄糖-6-磷酸脱氢酶(G6PD)的转录,该酶在肝脏再生过程中增强。过表达G6PD可恢复BMAL1-或hif -1α-缺陷肝脏受损的再生能力。此外,通过基因或术前间歇性禁食提高G6PD表达能有效促进正常小鼠的肝脏修复。因此,我们的研究结果强调了肝时钟的生理重要性,并提出了一种有希望的促再生策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


