Dirhodium–Palladium Dual-Catalyzed [1 + 1 + 3] Annulation to Heterocycles Using Primary Amines or H2O as the Heteroatom Sources
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The ever-increasing demand in chemical biology and medicinal research requires the development of new synthetic methods for the rapid construction of libraries of heterocycles from simple raw materials. In this context, the utilization of primary amines or H2O as the simple N- or O-sources in the assembly of a heterocyclic ring skeleton is highly desirable from the viewpoint of atom- and step-economy. Herein, we describe a highly efficient three-component reaction of diazo, allylic diacetates, and commercially available anilines (or H2O) to access structurally diverse pyrrolidine and tetrahydrofuran derivatives. This formal [1 + 1 + 3] annulation reaction features high efficiency, good yields, and broad functional group compatibility, making it a versatile and robust platform for the (formal) synthesis of several important bioactive molecules. Mechanistic studies suggested that the dirhodium–palladium bimetallic relay catalysis should play a key role in the successive steps of the current reaction, including sequential carbene insertion into the X–H bond and double allylic substitutions, thus allowing for building up molecular complexity from these simple raw materials.
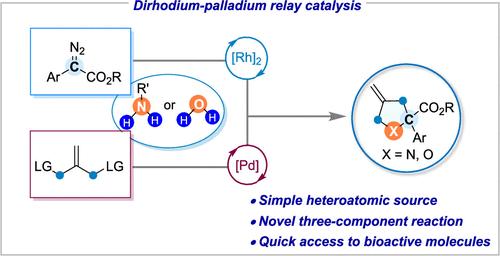
以伯胺或水为杂原子源的双催化[1 + 1 + 3]环化制备杂环
化学生物学和药物学研究的需求日益增长,这就需要开发新的合成方法,利用简单的原材料快速构建杂环化合物库。在这种情况下,从原子和步骤经济性的角度来看,利用伯胺或 H2O 作为组装杂环骨架的简单 N- 或 O-源是非常可取的。在此,我们介绍了重氮、烯丙基二乙酸酯和市售苯胺(或 H2O)的高效三组分反应,以获得结构多样的吡咯烷和四氢呋喃衍生物。这种正式的[1 + 1 + 3]环化反应具有高效率、高产率和广泛的官能团兼容性等特点,使其成为(正式)合成多种重要生物活性分子的通用而稳健的平台。机理研究表明,铑钯双金属接力催化应在当前反应的连续步骤中发挥关键作用,包括碳烯依次插入 X-H 键和双烯丙基取代,从而可以从这些简单的原料中建立复杂的分子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


