Triphenylphosphine Oxide-Derived Anolyte for Application in Nonaqueous Redox Flow Battery
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
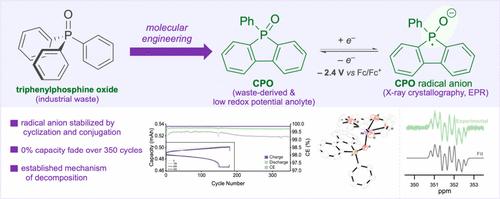
Recent advances in redox flow batteries have made them a viable option for grid-scale energy storage, however they exhibit low energy density. One way to boost energy density is by increasing the cell potential using a nonaqueous system. Molecular engineering has proven to be an effective strategy to develop redox-active compounds with extreme potentials but these are usually challenged by resource sustainability of the newly developed redox materials. Here, we investigate the utility of phosphine oxides as anolytes with extremely negative potentials. Specifically, we found that cyclic triphenylphosphine oxide (CPO), has a highly negative potential (−2.4 V vs Fc/Fc+). Importantly, CPO is synthesized from triphenylphosphine oxide, a common industrial chemical waste with no commercial value. Structural and electrochemical characterization of the reduced radical anion showed that enhanced stability is due to cyclization or extended pi-conjugation. Importantly, mechanistic investigation into the decomposition of CPO under various solvents and electrochemical conditions allowed us to utilize an acetonitrile/DMF binary solvent system to enable a long-lived anolyte which exhibited no fade over 350 cycles. In summary, this work led to the development of a waste-derived cyclic phosphine oxide that exhibits excellent cycling stability making it an ideal anolyte toward the development of energy-dense RFBs.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


