Intraosseous basivertebral nerve ablation: A 5-year pooled analysis from three prospective clinical trials
引用次数: 0
Abstract
Background
Vertebrogenic pain is a documented source of anterior column chronic low back pain (CLBP) that stems from damaged vertebral endplates. Nociceptive signals are transmitted by the basivertebral nerve (BVN) and endplate damage is observed as Type 1 or Type 2 Modic changes (MC) on magnetic resonance imaging (MRI). The clinical impact and safety of intraosseous radiofrequency ablation of the BVN (BVNA) for the treatment of vertebrogenic pain has been demonstrated in three prospective clinical trials (two randomized and one single-arm study).
Objective
Report aggregate long-term BVNA outcomes at five years from three studies.
Methods
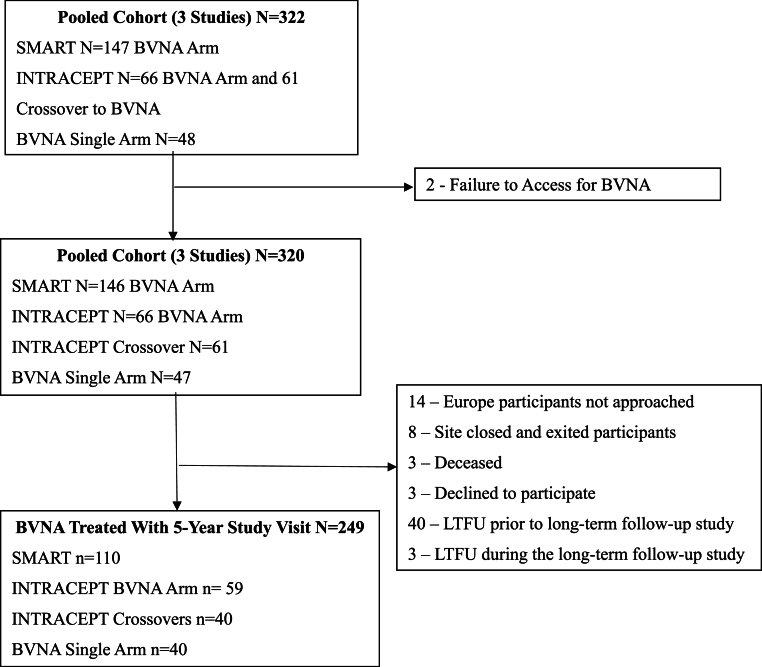
Pooled results at 5-years post-BVNA are reported for three clinical trials with similar inclusion/exclusion criteria and outcomes measurements: 1) a prospective, open label, single-arm follow-up of the treatment arm of a randomized controlled trial (RCT) comparing BVNA to sham ablation (SMART); 2) a prospective, open label, single-arm follow-up of the treatment arm of an RCT comparing BVNA to standard care (INTRACEPT); and 3) a prospective, open label, single-arm long-term follow-up study of BVNA-treated participants (CLBP Single-Arm). Paired datasets (baseline and 5-years) for mean changes in Oswestry disability index (ODI) and numeric pain scores (NPS) were analyzed using a two-sided paired t-test with a 0.05 level of significance. Secondary outcomes included responder rates, patient satisfaction, adverse events, and healthcare utilization.
Results
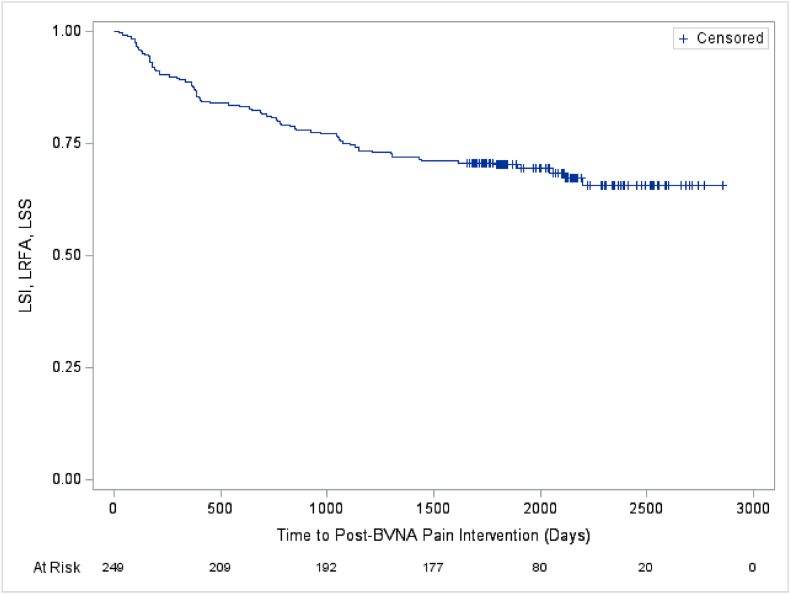
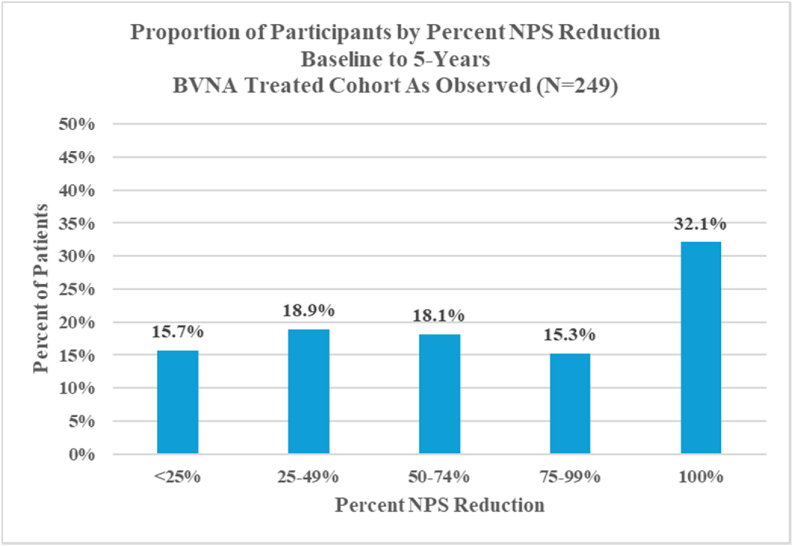
Two hundred forty-nine (249) of 320 BVNA-treated participants (78 % participation rate) completed a five-year visit (mean of 5.6 years follow-up). At baseline, 71.9 % of these participants reported back pain for ≥5 years, 27.7 % were taking opioids, and 61.8 % had prior therapeutic lumbar spinal injections. Pain and functional improvements were significant at 5-years with a mean improvement in NPS of 4.32 ± 2.45 points (95 % CI 4.01, 4.63; p < 0.0001) from 6.79 ± 1.32 at baseline and a mean improvement in ODI of 28.0 ± 17.5 (95 % CI 25.8, 30.2; p < 0.0001) from 44.5 ± 11.0 at baseline. Nearly one-third (32.1 %) of patients reported being pain-free (NPS = 0) at five years, 72.7 % of patients indicated their condition improved and 68.7 % had resumed activity levels they had prior to onset of CLBP. In the sixty-nine participants taking opioids at baseline, 65.2 % were no longer taking them at 5-years, and spinal injections decreased by 58.1 %. The rate of lumbosacral treatment (therapeutic spinal injection, radiofrequency ablation, or surgery) for the same index pain source and vertebral level was 33/249 (13.2 %) at 5 years post BVNA; including a 6.0 % rate of lumbar fusion. There were no serious device or device-procedure related adverse events reported during the long-term follow-up.
Conclusion
In this 5-year aggregate analysis, BVNA significantly improved pain and function scores compared to baseline. Similarly, there were significant reductions in opioid consumption and spinal injections post BVNA. Data demonstrate a strong safety profile with no serious device or device-related events and low healthcare utilization rate for the same index pain source through a mean of 5.6 years. Results demonstrate that intraosseous BVNA treatment for patients with vertebrogenic pain is safe, effective, and durable through five years.
骨内椎体神经消融:一项来自三个前瞻性临床试验的5年汇总分析。
背景:椎体源性疼痛是由椎体终板损伤引起的前柱慢性腰痛(CLBP)的一种有文献记载的来源。痛觉信号由椎基神经(BVN)传递,终板损伤在磁共振成像(MRI)上表现为1型或2型moic改变(MC)。骨内射频消融BVNA (BVNA)治疗椎体源性疼痛的临床影响和安全性已在三项前瞻性临床试验(两项随机研究和一项单臂研究)中得到证实。目的:从三项研究中报告5年BVNA的总体长期结果。方法:报告了BVNA后5年的三个临床试验的汇总结果,它们具有相似的纳入/排除标准和结果测量:1)一项比较BVNA和假消融(SMART)的随机对照试验(RCT)治疗组的前瞻性、开放标签、单臂随访;2)一项比较BVNA与标准治疗(INTRACEPT)的RCT治疗组的前瞻性、开放标签、单臂随访;3) bvna治疗参与者的前瞻性、开放标签、单臂长期随访研究(CLBP单臂)。配对数据集(基线和5年)对Oswestry残疾指数(ODI)和数值疼痛评分(NPS)的平均变化进行分析,采用双侧配对t检验,显著性水平为0.05。次要结局包括应答率、患者满意度、不良事件和医疗保健利用。结果:320名接受bvna治疗的参与者中249人(78%的参与率)完成了5年的随访(平均随访5.6年)。在基线时,71.9%的参与者报告背痛≥5年,27.7%服用阿片类药物,61.8%之前接受过腰椎注射治疗。5年疼痛和功能改善显著,NPS平均改善4.32±2.45分(95% CI 4.01, 4.63;结论:在这项5年的综合分析中,与基线相比,BVNA显著改善了疼痛和功能评分。同样,BVNA后阿片类药物的消耗和脊柱注射也显著减少。数据表明,在平均5.6年的时间里,没有严重的器械或器械相关事件,同一指数疼痛源的医疗利用率很低,安全性很强。结果表明骨内BVNA治疗椎体源性疼痛是安全、有效和持续5年的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


