Monitoring of Circulating Tumor DNA and Indication of De-Escalation Adjuvant Targeted Therapy for EGFR-Mutated NSCLC After Complete Resection
IF 3.5
Q2 ONCOLOGY
引用次数: 0
Abstract
Introduction
EGFR tyrosine kinase inhibitor (TKI) is the standard adjuvant treatment for patients with stages IB to IIIA EGFR-mutated NSCLC. Nevertheless, adapting this approach to include a molecular residual disease (MRD)-guided de-escalation strategy warrants further investigation.
Methods
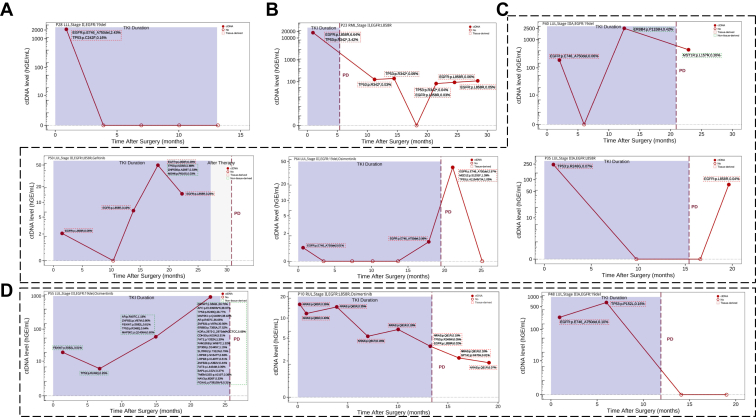
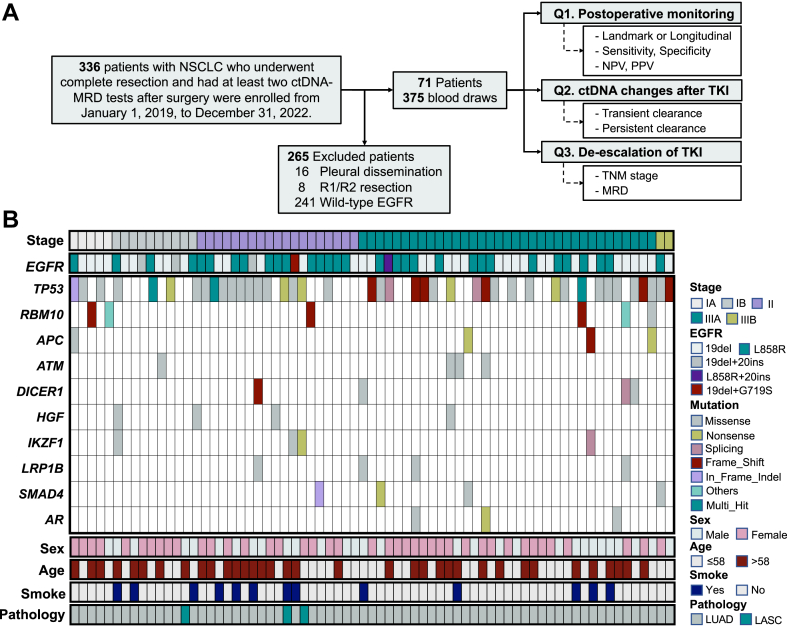
From January 2019 to December 2022, 71 patients with stages I to III NSCLC and EGFR (exon 19 deletion or L858R) mutations were enrolled in this observational study. A total of 375 blood samples were analyzed using the MRD_Navigator assay. Among them, 27 patients suspended EGFR TKI treatment based on undetectable MRD and were thus included in the adaptive, de-escalation group.
Results
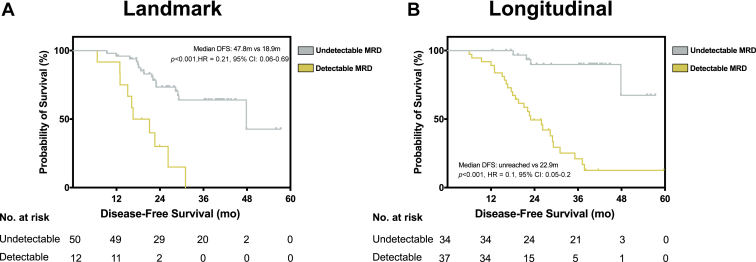
Overall, the sensitivity of longitudinal MRD was 86.2%. Only four patients (11.8%) recurred with longitudinal undetectable MRD, indicating a negative predictive value of 88.2%. Of the patients who had detectable MRD after surgery, nine subsequently received EGFR TKI treatment, with only one (11.1%) achieving persistent circulating tumor DNA clearance post–EGFR TKI. Furthermore, 22 patients with stages IB to III disease who had previously suspended their TKI treatment based on undetectable MRD were included in the adaptive group, with an average duration of TKI 3.9 (range: 0–35.0) months. The 2-year disease-free survival rate of these 22 patients was 80.2%, and the median was not reached. Five patients (n = 5 of 22, 22.7%) had disease recurrence during the period of drug cessation but were stable under EGFR TKI treatment until the latest follow-up. Two patients remained in complete remission.
Conclusions
Our initial findings underscore the potential of an adaptive, de-escalation approach to adjuvant EGFR TKIs based on circulating tumor DNA-MRD monitoring.
egfr突变的非小细胞肺癌完全切除后循环肿瘤DNA的监测和降级辅助靶向治疗的适应症。
简介:EGFR酪氨酸激酶抑制剂(TKI)是IB至IIIA期EGFR突变的NSCLC患者的标准辅助治疗。然而,将这种方法纳入分子残留病(MRD)引导的降级策略需要进一步的研究。方法:从2019年1月到2022年12月,71例I至III期NSCLC和EGFR(外显子19缺失或L858R)突变的患者入组了这项观察性研究。使用MRD_Navigator检测共分析了375份血液样本。其中,27例患者因MRD检测不出而暂停EGFR TKI治疗,因此被纳入适应性降糖组。结果:总体而言,纵向MRD的敏感性为86.2%。只有4名患者(11.8%)复发,纵向无法检测MRD,阴性预测值为88.2%。在术后MRD检测到的患者中,9例随后接受了EGFR TKI治疗,只有1例(11.1%)在EGFR TKI后实现了持续的循环肿瘤DNA清除。此外,22名先前因MRD检测不出而暂停TKI治疗的IB至III期疾病患者被纳入适应性组,TKI平均持续时间为3.9个月(范围:0-35.0)。这22例患者的2年无病生存率为80.2%,未达到中位数。5例患者(n = 5 / 22,22.7%)在停药期间疾病复发,但在EGFR TKI治疗下病情稳定,直到最近一次随访。两名患者仍处于完全缓解状态。结论:我们的初步研究结果强调了基于循环肿瘤DNA-MRD监测的EGFR TKIs辅助治疗的适应性、降级方法的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


