Interfacial Properties of Gold and Cobalt Oxyhydroxide in Plasmon-Mediated Oxygen Evolution Reaction
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
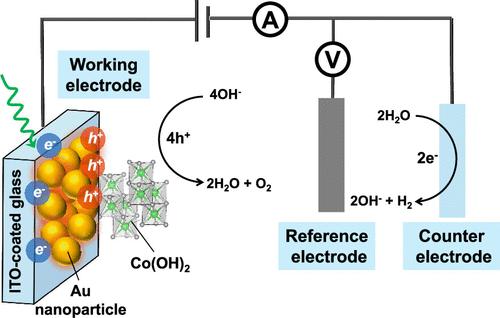
Water electrolysis is a green method of storing electrical energy in the chemical bonds of high-energy hydrogen gas (H2). However, the anodic oxygen evolution reaction (OER) requires a significant kinetic overpotential, limiting the electrolysis rate. Recently, plasmonic gold nanoparticles (Au NPs) have been introduced to improve charge transfer at the interface between the OER electrocatalysts and the electrolyte under light illumination. Despite this, the mechanism by which Au NPs enhance photoassisted electrochemical processes remains poorly understood. To address this, we employed a model system comprising a plasmonic Au electrode and a cobalt (Co)-based electrocatalyst in alkaline electrolytes, studying the plasmon-mediated OER process through (photo)electrochemical and spectroscopic methods. Our findings revealed that a surfactant-free, electrodeposited plasmonic Au electrode could significantly enhance the electrocatalytic performance of Co-based OER electrocatalysts under continuous visible and near-infrared light illumination. Transient photocurrent studies showed that both the photothermal effect and energetic charge carriers contributed to the improved OER performance, with the Au|Co catalyst interface playing a key role in these enhancements. Additionally, electrochemical Raman measurements identified the active phase of the Co-based OER electrocatalyst to be cobalt oxyhydroxide (CoOOH) at oxidizing potentials.
等离子体介导的析氧反应中金和钴的界面性质
水电解是一种将电能储存在高能氢气(H2)化学键中的绿色方法。然而,阳极析氧反应(OER)需要显著的动力学过电位,限制了电解速率。近年来,等离子体金纳米粒子(Au NPs)被引入到OER电催化剂和电解质之间的界面上,以改善光照下的电荷转移。尽管如此,Au NPs增强光辅助电化学过程的机制仍然知之甚少。为了解决这个问题,我们采用了一个由等离子体Au电极和碱性电解质中的钴基电催化剂组成的模型系统,通过(照片)电化学和光谱方法研究了等离子体介导的OER过程。我们的研究结果表明,在连续可见光和近红外光照射下,无表面活性剂的电沉积等离子体Au电极可以显著提高co基OER电催化剂的电催化性能。瞬态光电流研究表明,光热效应和高能载流子都有助于提高OER性能,Au|Co催化剂界面在这些增强中起着关键作用。此外,电化学拉曼测量确定了co基OER电催化剂在氧化电位下的活性相为氧化钴(CoOOH)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


