One-Dimensional Indium(III) Halide Double Perovskites (CH3NH3)2NaInX6 (X = Cl, Br) and Their Antimony(III)-Induced High Photoluminescence
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
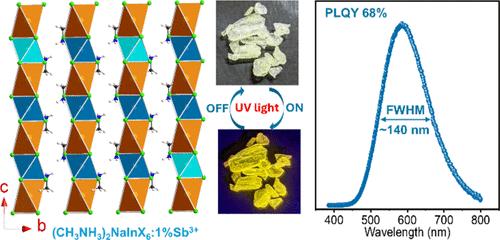
Low-dimensional In(III) halide perovskites have become one of the most attractive classes of light-emitting materials due to their tunable and high photoluminescence efficiency. However, their synthesis is still a challenge. Here, we report two novel Na(I)–In(III) halide double perovskite-related compounds (MA)2NaInCl6 and (MA)2NaInBr6 and their Sb3+-doped counterparts. Both compounds crystallize in one-dimensional (1D) face-sharing chain structures with a trigonal P3̅m1 symmetry. (MA)2NaInCl6 and (MA)2NaInBr6 show wide and direct band gaps of 5.3 and 3.9 eV, respectively. While both materials are nonemissive in their pristine forms, 5% Sb3+-doped (MA)2NaInCl6 and (MA)2NaInBr6 show green (555 nm) and yellow (585 nm) emission with the photoluminescence quantum yields of 13.8 and 53.6%, respectively. For (MA)2NaInBr6, a PLQY of 67.64% was achieved with 1% Sb doping. Variable-temperature PL studies and density functional theory calculations indicate that the Sb3+ ion introduces self-trapped excitonic (STE) states, which are responsible for the high-efficiency PL emission. Our findings significantly expand the scope of halide double perovskites to low-dimensional photoluminescent In(III)-based metal halide perovskites.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


