Area-Independent Approach for Measuring Electrosorption Kinetics without Assuming Specific Isotherm Models: Application to Hydrogen Underpotential Deposition
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Despite significant advancements in electrochemical techniques, kinetic measurements in electrocatalysis still predominantly rely on voltammetric curves and Tafel plots, which can lead to data misinterpretation and overestimation of catalytic efficiency due to area effects. Addressing this gap, this article introduces a staircase voltammetry-based method to investigate the kinetics of electrosorption. By leveraging the pulsed nature of staircase voltammetry, this approach enables the determination of rate constants independent of real electrode surface area, using a single voltammetric cycle. The method was applied to study hydrogen underpotential deposition (HUPD) on polycrystalline platinum using electrodes with varying roughness. Thermodynamic and kinetic parameters were determined for the two voltammetric peaks. Furthermore, the underlying theory of the method is thoroughly presented, facilitating its extension to other systems.
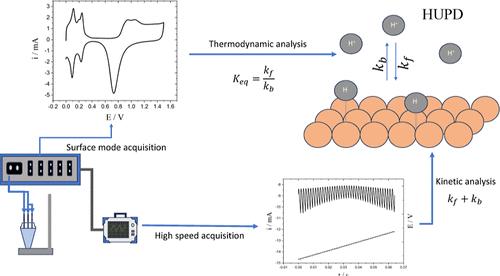
测量电吸附动力学的面积无关方法,而不假设特定的等温线模型:应用于氢欠电位沉积
尽管电化学技术取得了重大进展,但电催化的动力学测量仍然主要依赖于伏安曲线和Tafel图,这可能导致数据误解和由于面积效应而高估催化效率。为了解决这一问题,本文介绍了一种基于阶梯伏安法的方法来研究电吸附动力学。通过利用阶梯伏安法的脉冲性质,这种方法可以确定独立于实际电极表面积的速率常数,使用单个伏安循环。应用该方法研究了不同粗糙度电极在多晶铂上的氢欠电位沉积(HUPD)。测定了两个伏安峰的热力学和动力学参数。此外,还详细介绍了该方法的基本原理,便于将其推广到其他系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


