Electrochemical Reduction of CO2 to CH3OH Catalyzed by an Iron Porphyrinoid
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Designing catalysts for the selective reduction of CO2, resulting in products having commercial value, is an important area of contemporary research. Several molecular catalysts have been reported to facilitate the reduction of CO2 (both electrochemical and photochemical) to yield 2e–/2H+ electron-reduced products, CO and HCOOH, and selective reduction of CO2 beyond 2e–/2H+ is rare. This is partly because the factors that control the selectivity of CO2 reduction beyond 2e– are not yet understood. An iron chlorin complex with a pendent amine functionality in its second sphere, known to selectively catalyze CO2RR to HCOOH with a very low overpotential from its formal Fe(I) state, can catalyze CO2RR from its formal Fe(0) state by 6e–/6H+, forming CH3OH as a major product with a Faradaic yield of ∼50%. Mechanistic investigations using in situ spectro-electrochemistry indicate that the reactivity of a low-spin d7 FeI–COOH intermediate species generated during CO2RR is crucial in determining the product selectivity of this reaction. In weakly acidic conditions, C-protonation of this FeI–COOH species, which is also chemically prepared and spectroscopically characterized, leads to HCOOH. The O-protonation, leading to C–OH bond cleavage and eventually to CH3OH, is ∼3 kcal/mol higher in energy and can be achieved in more acidic solutions. Hydrogen bonding to the pendent amine in the catalyst stabilizes reactive intermediates formed in the CO2RR and enables 6e–/6H+ reduction of CO2 to CH3OH.
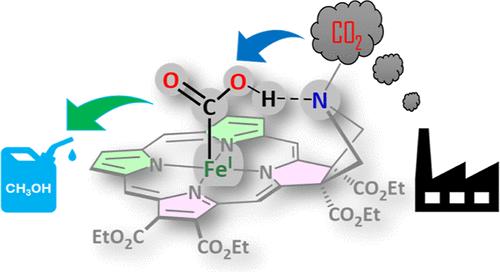
铁卟啉类催化CO2电化学还原为CH3OH
设计选择性还原CO2的催化剂,从而产生具有商业价值的产品,是当代研究的一个重要领域。据报道,几种分子催化剂可以促进CO2的还原(电化学和光化学),生成2e - /2H+电子还原产物CO和HCOOH,而选择性还原超过2e - /2H+的CO2是罕见的。这在一定程度上是因为控制二氧化碳减排选择性的因素尚不清楚。铁氯配合物在其第二球中具有悬置胺功能,已知可以选择性地催化CO2RR以极低的过电位从其形式的Fe(I)态转化为HCOOH,可以通过6e - /6H+催化CO2RR从其形式的Fe(0)态转化为CH3OH,作为主要产物,法拉第产率为~ 50%。利用原位光谱电化学的机理研究表明,在CO2RR过程中产生的低自旋d7 FeI-COOH中间物质的反应活性是决定该反应产物选择性的关键。在弱酸性条件下,这种FeI-COOH的c -质子化,也被化学制备和光谱表征,导致HCOOH。导致C-OH键断裂并最终生成CH3OH的o -质子化反应的能量要高~ 3 kcal/mol,并且可以在酸性更强的溶液中实现。催化剂中与悬垂胺的氢键稳定了CO2RR中形成的反应中间体,并使6e - /6H+将CO2还原为CH3OH。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


