Lipophilicity Modulation of Fluorescent Probes for In Situ Imaging of Cellular Microvesicle Dynamics
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
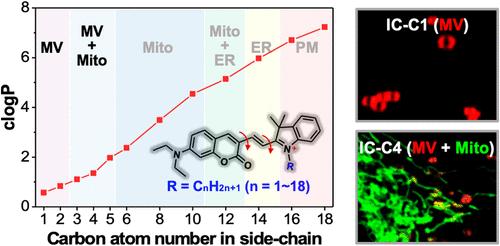
Real-time monitoring of dynamic microvesicles (MVs), vesicles associated with living cells, is of great significance in deeply understanding their origin, transport, and function. However, specific labeling MVs poses a challenge due to the lack of unique biomarkers that differentiate them from other cellular compartments. Here, we present a strategy to selectively label MVs by evaluating a series of lipid layer-sensitive cationic indolium-coumarin fluorescent probes (designated as IC-Cn, with n ranging from 1 to 18) that feature varying aliphatic side chains (CnH2n+1). Through in situ cell imaging and analysis, we found that IC-Cn location is highly related to their lipophilicities and the phospholipid layer hydrophobic microenvironments in cellular compartments. In detail, IC-C1 and IC-C2 specifically localize MVs both inside and outside cells. In contrast, IC-C3, IC-C4, and IC-C5 label cellular MVs and mitochondria but with distinct fluorescence lifetimes. Using these probes strategically, we have discovered that, in addition to the biogenesis of MVs from plasma membranes and damaged mitochondria, newly formed MVs can undergo fusion and fission processes. Moreover, mitochondria-derived MVs, beyond being released from parent cells, can fuse with lysosomes to facilitate the removal of dysfunctional mitochondria. The work not only provides new insights into MV physiology but also inspires the design strategies for probes used in specific labeling in cell studies.
细胞微囊动力学原位成像荧光探针的亲脂性调制
动态微囊泡(MVs)是与活细胞相关的囊泡,对其进行实时监测对于深入了解其起源、运输和功能具有重要意义。然而,由于缺乏将其与其他细胞区室区分开来的独特生物标志物,特异性标记mv带来了挑战。在这里,我们提出了一种通过评估一系列脂质层敏感阳离子吲哚-香豆素荧光探针(指定为IC-Cn, n范围从1到18)来选择性标记mv的策略,这些探针具有不同的脂肪侧链(CnH2n+1)。通过原位细胞成像和分析,我们发现IC-Cn的位置与它们的亲脂性和细胞室内磷脂层疏水微环境高度相关。IC-C1和IC-C2特异定位细胞内外的mv。相比之下,IC-C3, IC-C4和IC-C5标记细胞mv和线粒体,但具有不同的荧光寿命。策略性地使用这些探针,我们发现,除了来自质膜和受损线粒体的MVs的生物发生外,新形成的MVs可以经历融合和裂变过程。此外,线粒体来源的mv,除了从亲本细胞中释放外,还可以与溶酶体融合,以促进功能失调线粒体的清除。这项工作不仅为MV生理学提供了新的见解,而且还启发了用于细胞研究中特定标记的探针的设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


