Reactions of Methyl Radicals with Iridium, Osmium, and Ruthenium Nanoparticles Suspended in Aqueous Solutions: Surface Effects
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The reactions of methyl radicals with noble metal nanoparticles, M0-NPs, M = Ru, Os, and Ir, were studied. The M0-NPs were prepared by reduction of the corresponding salts with NaBH4. HR-TEM, XPS, and Raman analysis of the M0-NPs thus formed point out that the Ru0-NPs and Os0-NPs formed are covered with an oxide/hydroxide layer. The Ir0-NPs are only partially covered by such a layer. The methyl radicals oxidize all of the M0-NPs studied herein. Thus, the mechanism differs from the mechanisms of reaction with M0-NPs, M = Ag, Au, and Pt. This phenomenon is attributed to the differences in the reduction potentials of these metals. The rate constant of the reaction of methyl radicals with Ir0-NP is very high, similar to that reported for other M0-NPs, M = Ag, Au, and Pt. This is attributed to the interaction of the radicals with the electrons in the conduction bands of the metals. On the other hand, the rate constants of the reactions of methyl radicals with M0-NPs, M = Ru and Os, are considerably lower and similar to those reported for titania. The total yield of methyl radicals, the yield of methane + twice the yield of ethane, observed in the presence of the M0-NPs, is somewhat smaller than expected. This is attributed to either the formation of a mixture of side products, at concentrations below the limit of observation under our experimental conditions, or some energy absorption by the M0-NPs.
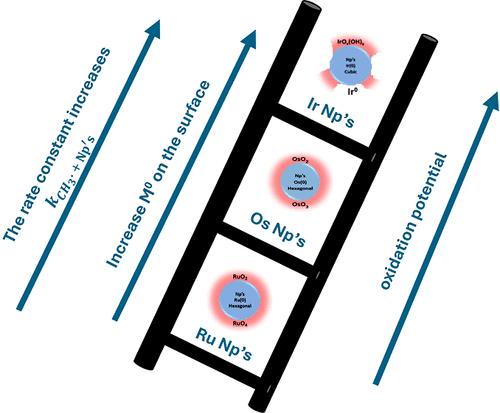
甲基自由基与悬浮在水溶液中的铱、锇和钌纳米粒子的反应:表面效应
研究了甲基自由基与贵金属纳米粒子M0-NPs、M = Ru、Os和Ir的反应。用NaBH4还原相应的盐制备M0-NPs。对形成的M0-NPs的HR-TEM、XPS和拉曼分析表明,形成的Ru0-NPs和o0 - nps表面覆盖有氧化/氢氧化物层。ir0 - np仅部分被这一层覆盖。甲基自由基氧化本文研究的所有M0-NPs。因此,该机理不同于与M0-NPs、M = Ag、Au和Pt的反应机理。这种现象归因于这些金属的还原电位的差异。甲基自由基与Ir0-NP的反应速率常数非常高,与其他m0 - np, M = Ag, Au和Pt的反应速率常数相似。这是由于自由基与金属导电带中的电子相互作用所致。另一方面,甲基自由基与M0-NPs (M = Ru和Os)的反应速率常数较低,与二氧化钛的反应速率常数相似。在M0-NPs存在下,甲基自由基的总产率(甲烷+乙烷产率的两倍)略小于预期。这是由于在我们的实验条件下,在浓度低于观察极限的情况下,形成了副产物的混合物,或者是由于M0-NPs吸收了一些能量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


