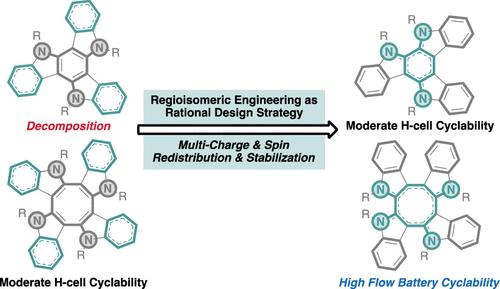
Regioisomeric Engineering for Multicharge and Spin Stabilization in Two-Electron Organic Catholytes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Developing multicharge and spin stabilization strategies is fundamental to enhancing the lifetime of functional organic materials, particularly for long-term energy storage in multiredox organic redox flow batteries. Current approaches are limited to the incorporation of electronic substituents to increase or decrease the overall electron density or bulky substituents to sterically shield reactive sites. With the aim to further expand the molecular toolbox for charge and spin stabilization, we introduce regioisomerism as a scaffold-diversifying design element that considers the collective and cumulative electronic and steric contributions from all of the substituents based on their relative regioisomeric arrangements. Through a systematic study of regioisomers of near-planar aromatic cyclic triindoles and nonplanar nonaromatic cyclic tetraindoles, we demonstrate that this regioisomeric engineering strategy significantly enhances the H-cell cycling stability in the above two new classes of 2e– catholytes, even when current strategies failed to stabilize the multicharged species. Density functional theory calculations reveal that the strategy operates by redistributing the charge and spin densities while highlighting the role of aromaticity in charge stabilization. The most stable 2e– catholyte candidate was paired with a viologen derivative anolyte to achieve a proof-of-concept all-organic flow battery with 1.26–1.49 V, 98% capacity retention, and only 0.0117% fade/h and 0.00563% fade/cycle over 400 cycles (192 h), which is the highest capacity retention ever reported over 400 cycles in a multielectron all-organic flow battery setup. We anticipate regioisomeric engineering to be a promising strategy complementary to conventional electronic and steric approaches for multicharge and spin stabilization in other functional organic materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


