Photoinduced Regiodivergent and Enantioselective Cross-Coupling of Glycine Derivatives with Hydrocarbon Feedstocks
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Regiodivergent asymmetric synthesis represents a transformative strategy for the efficient generation of structurally diverse chiral products from a single set of starting materials, significantly enriching their enantiomeric composition. However, the design of radical-mediated regiodivergent and enantioselective reactions that can accommodate a wide range of functional groups and substrates has posed significant challenges. The obstacles primarily lie in switching the regioselectivity and achieving high enantiodiscrimination, especially when dealing with high-energy intermediates. To address these issues, we have developed a new catalytic system that integrates photoinduced hydrogen atom transfer (HAT) and chiral copper catalysis, involving the fine-tuning of chiral ligands, additives, and other reaction parameters. The strategy facilitates regiodivergent and enantioselective cross-couplings between N-aryl glycine ester/amide derivatives and abundant hydrocarbon feedstocks through strong C(sp3)–H bond activation. This approach allows for the controlled and stereoselective formation of C(sp3)–C(sp3) and C(sp3)–N bonds, yielding a rich variety of C- or N-alkylated glycine esters and amides with commendable yields (up to 92% yield), exclusive regioselectivities (typically >20:1 rr), and high enantioselectivities (up to 96% ee). Our methodology not only provides a promising avenue for the stereoselective incorporation of alkyl functionalities onto specific sites of biologically significant molecules but also offers a practical approach for regioselectivity switching while simultaneously achieving high asymmetric induction within photochemical reactions.
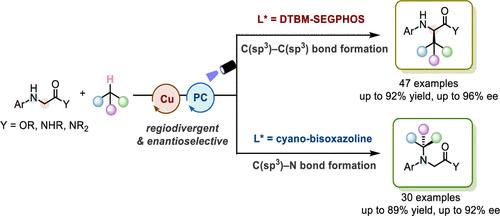
甘氨酸衍生物与烃类原料的光诱导区域发散和对映选择性交叉偶联
区域发散不对称合成代表了一种革命性的策略,从一组起始材料高效地生成结构多样的手性产物,显著丰富了它们的对映体组成。然而,自由基介导的区域发散和对映选择性反应的设计,可以适应广泛的功能基团和底物已经提出了重大的挑战。障碍主要在于改变区域选择性和实现高对映分辨,特别是在处理高能中间体时。为了解决这些问题,我们开发了一种新的催化系统,该系统集成了光诱导氢原子转移(HAT)和手性铜催化,涉及手性配体、添加剂和其他反应参数的微调。该策略通过强烈的C(sp3) -H键激活,促进n -芳基甘氨酸酯/酰胺衍生物与丰富的烃类原料之间的区域发散性和对映选择性交叉偶联。这种方法允许控制和立体选择性形成C(sp3) - C(sp3)和C(sp3) - n键,产生丰富的C或n烷基化甘氨酸酯和酰胺,具有良好的收率(高达92%收率),独家区域选择性(通常为20:1 rr)和高对映选择性(高达96% ee)。我们的方法不仅为烷基官能团立体选择性结合到具有生物学意义的分子的特定位点提供了一条有希望的途径,而且为区域选择性切换提供了一种实用的方法,同时在光化学反应中实现了高不对称诱导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


