Atomic-Scale In Situ Self-Catalysis Growth of Graphite Shells via Pyrolysis of Various Metal Phthalocyanines
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
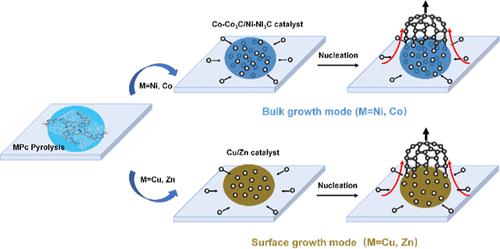
Carbon nanomaterials, as novel functional and structural materials with unique properties, hold significant potential in information technology and nanodevices. However, the self-catalytic growth mechanism of graphite shells derived from carbon sources, facilitated by diverse catalysts, necessitates further exhaustive investigation. To tackle this challenge, we conducted a study utilizing environmental transmission electron microscopy (ETEM) at atomic resolution to investigate the pyrolysis of metal phthalocyanine (MPc) compounds containing various central metals, including Ni, Co, Cu, and Zn. The results reveal that carbon atoms initially dissolve into Ni and Co, leading to the formation of metal carbides that subsequently act as catalysts. Upon achieving carbon saturation, these atoms precipitate outward from the interior, fostering the growth of graphite shells through a bulk growth mode. In contrast, for Cu and Zn, carbon atoms migrate and accumulate on the metal surface, resulting in the growth of graphite shells via a surface growth mode. The distinct growth mechanisms, i.e., bulk growth and surface growth, are intricately governed by the binding energy between carbon atoms and the catalysts, as well as the carbon solubility in the metal. These factors are closely tied to the number and energy of the metals’ d-electrons because more vacant d-orbitals easily forming more coordination numbers. These insights offer profound understanding into the fundamental principles of metal-catalyzed graphite shell growth and provide a strategic roadmap for selecting and designing catalysts that can can optimize the synthesis of desirable carbon nanomaterials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


