Metal–Ligand Spin-Lock Strategy for Inhibiting Anion Dimerization in Li-Rich Cathode Materials
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
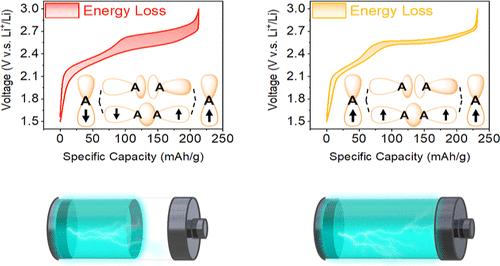
Anion dimerization poses a significant challenge for the application of Li-rich cathode materials (LCMs) in high-energy-density Li-ion batteries because of its deleterious effects, including rapid capacity and voltage decay, sluggish reaction kinetics, and large voltage hysteresis. Herein, we propose a metal–ligand spin-lock strategy to inhibit anion dimerization, which involves introducing an Fe–Ni couple having antiferromagnetic superexchange interaction into the LCM to lock the spin orientations of the unpaired electrons in the anions in the same direction. As proof of concept, we applied this strategy to intralayer disordered Li2TiS3 (ID-LTS) to inhibit S–S dimerization. Electrochemical characterization using the galvanostatic charge/discharge and intermittent titration technique demonstrated the considerably enhanced anionic redox activity, reduced voltage hysteresis, and improved kinetics of the Fe–Ni-couple-incorporated ID-LTS. Fe L2,3-edge X-ray absorption spectroscopy and magnetic susceptibility measurements revealed that the metal–ligand spin-lock effect and consequent suppression of anion dimerization involve ligand-to-metal charge transfer between S and Fe. Further electrochemical tests on a Fe–Ni-couple-incorporated Li-rich layered oxide (Li0.7Li0.1Fe0.2Ni0.1Mn0.6O2) indicated the importance of the π backbond in enhancing ligand-to-metal charge transfer from S to Fe. These findings demonstrate the potential application of our metal–ligand spin-lock strategy in the development of high-performance LCMs.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


