Prediction of Methylcyclopentane-Shaped N6 Building Bolcks and Fused N22 Macro-Rings in Potassium Chloride Nitrogen Salts at High Pressure
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Polymeric nitrogen has garnered significant research interest due to its unique physicochemical properties and substantial potential as a propellant. Tailored ionic compounds, as emerging inducers, actively enrich the topology of polymeric nitrogen frameworks by enhancing their stability and reactivity through synergistic interactions. Here, we identify the pressure-stabilized methylcyclopentane-shaped N6 building blocks and fused N22 macro-rings in thermodynamically stable KClN6 and KClN10 compounds, respectively, employing swarm-intelligence structure prediction methodology and first-principles calculations. Notably, differing from known N6 benzene rings found in metal hexanitrides, the unique arrangement of the methylcyclopentane-shaped N6 building block is attributed to the dual actions of ionic and covalent interactions between Cl and nearby N atoms, which help maintain the integrity of the polymeric form. Ab initio molecular dynamics simulations and phonon spectra calculations demonstrated the potential retrieval of KClN6 as a metastable phase under atmospheric conditions. KClN6 exhibits desirable characteristics of high energy release, low mass density, high detonation velocity, and high detonation pressure, highlighting its potential as a high energy-density material. These findings provide a new route for the creation of polymeric nitrogen in customized ionic compounds and stimulate experimental search.
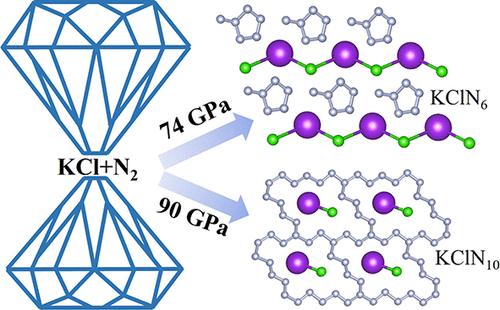
高压下甲基环戊烷型N6构筑块和熔融N22大环在氯化钾氮盐中的预测
聚合物氮由于其独特的物理化学性质和作为推进剂的巨大潜力而引起了人们极大的研究兴趣。定制离子化合物作为新兴的诱导剂,通过协同作用增强聚合物氮框架的稳定性和反应性,积极丰富聚合物氮框架的拓扑结构。本文采用群体智能结构预测方法和第一性原理计算,在热力学稳定的KClN6和KClN10化合物中分别鉴定了压力稳定的甲基环戊烷形状的N6构建块和融合的N22宏观环。值得注意的是,与已知的在金属六氮化合物中发现的N6苯环不同,甲基环戊烷形状的N6构建块的独特排列归因于Cl和附近N原子之间的离子和共价相互作用的双重作用,这有助于保持聚合物形式的完整性。从头算分子动力学模拟和声子谱计算证明了KClN6在大气条件下作为亚稳相的潜在恢复。KClN6表现出高能量释放、低质量密度、高爆速和高爆压的优良特性,突出了其作为高能量密度材料的潜力。这些发现为在定制离子化合物中生成聚合氮提供了新的途径,并刺激了实验研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


