O2 Activation and Enzymatic C–H Bond Activation Mediated by a Dimanganese Cofactor
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
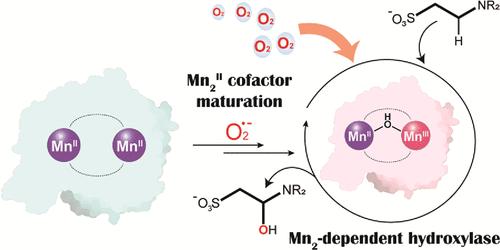
Dioxygen (O2) is a potent oxidant used by aerobic organisms for energy transduction and critical biosynthetic processes. Numerous metalloenzymes harness O2 to mediate C–H bond hydroxylation reactions, but most commonly feature iron or copper ions in their active site cofactors. In contrast, many manganese-activated enzymes─such as glutamine synthetase and isocitrate lyase─perform redox neutral chemical transformations and very few are known to activate O2 or C–H bonds. Here, we report that the dimanganese-metalated form of the cambialistic monooxygenase SfbO (Mn2–SfbO) can efficiently mediate enzymatic C–H bond hydroxylation. The activity of the dimanganese form of SfbO toward substrate hydroxylation is comparable to that of its heterobimetallic Mn/Fe form but exhibits distinct kinetic profiles. Kinetic, spectroscopic, and structural studies invoke a mixed-valent dimanganese cofactor (MnIIMnIII) in O2 activation and evidence a stoichiometric role for superoxide in maturing an O2-inert MnII2 cofactor. Computational studies support a hypothesis wherein superoxide addition to the MnII2 cofactor installs a critical bridging hydroxide ligand that stabilizes higher-valent manganese oxidation states. These findings establish the viability of proteinaceous dimanganese cofactors in mediating complex, multistep redox transformations.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


