Harnessing the Reactivity of Cyclopropenes in the Synthesis of Spiroketals via Putative Generation of Donor–Acceptor Cyclopropanes
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
TMSOTf-mediated 5/6-exo-trig hydroalkoxylation followed by the (3 + 2) cycloaddition cascade reaction of hydroxy cyclopropenes with aldehydes gave an expedient, stereoselective synthesis of [5.5]-and [6.5]-spiroketal derivatives. The developed protocol provides a new approach toward the synthesis of spiroketals from hydroxy cyclopropenes via a transiently generated donor–acceptor cyclopropane intermediate. These spirocyclic derivatives could be transformed to facilitate the access of intricate polycyclic heterocycles via a metal halogen exchange reaction and a copper-catalyzed C–O cross coupling reaction. The decarboxylation of the diester diastereoselectively sets the fourth chiral center of the spiroketal product.
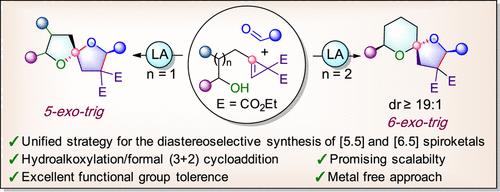
利用环丙烯在螺旋酮合成中的反应活性,通过假定生成给受体环丙烯
tmsotf介导的5/6-外三羟基羟基烷氧基化,然后是羟基环丙烯与醛的(3 + 2)环加成级联反应,方便地合成了[5.5]和[6.5]-螺旋酮衍生物。该方案为羟基环丙烯通过瞬态生成的供体-受体环丙烷中间体合成螺旋酮提供了一种新的途径。这些螺旋环衍生物可以通过金属卤素交换反应和铜催化的C-O交叉偶联反应转化成复杂的多环杂环。二酯的非对映选择性脱羧设置螺旋产物的第四个手性中心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


