Quantitative Proteomics Identifies Profilin-1 as a Pseudouridine-Binding Protein
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Pseudouridine (Ψ) is the most abundant RNA modification in nature; however, not much is known about the biological functions of this modified nucleoside. Employing an unbiased quantitative proteomics method, we identified multiple candidate reader proteins of Ψ in RNA, including a cytoskeletal protein profilin-1 (PFN1). We demonstrated that PFN1 binds directly and selectively to Ψ-containing RNA. Additionally, we discovered approximately 4000 binding sites of PFN1 in human cells, including a known dyskerin (DKC1)-installed Ψ site in TPI1 mRNA, which encodes triosephosphate isomerase. Moreover, we showed that PFN1 and DKC1 are crucial for regulating the stability and translation efficiency of TPI1 mRNA through modulating PFN1-Ψ interaction. Together, we identified PFN1 as a reader protein of Ψ in RNA and illustrated a potential role of PFN1-Ψ interaction in post-transcriptional regulation. These findings provide new insights into the functions of Ψ in RNA biology and in modulating the expression of an important metabolic enzyme.
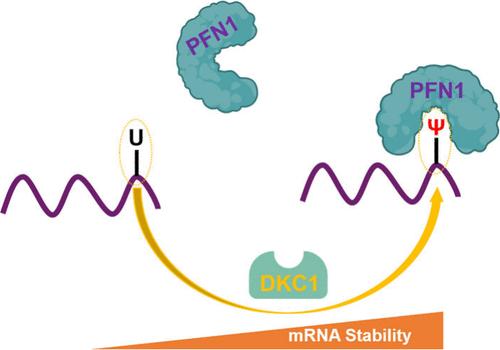
定量蛋白质组学鉴定Profilin-1为假尿嘧啶结合蛋白
伪尿嘧啶(Ψ)是自然界中最丰富的RNA修饰;然而,对这种修饰核苷的生物学功能所知不多。采用无偏定量蛋白质组学方法,我们确定了RNA中Ψ的多个候选解读蛋白,包括细胞骨架蛋白谱-1 (PFN1)。我们证明了PFN1直接和选择性地结合Ψ-containing RNA。此外,我们在人类细胞中发现了大约4000个PFN1结合位点,包括TPI1 mRNA中一个已知的dyskerin (DKC1)-安装Ψ位点,该位点编码三磷酸异构酶。此外,我们发现PFN1和DKC1通过调节PFN1-Ψ相互作用对调节TPI1 mRNA的稳定性和翻译效率至关重要。总之,我们确定了PFN1是RNA中Ψ的读取蛋白,并说明了PFN1-Ψ相互作用在转录后调控中的潜在作用。这些发现为Ψ在RNA生物学中的功能和调节一种重要代谢酶的表达提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


