Surface Oxygen Vacancies on Copper-Doped Titanium Dioxide for Photocatalytic Nitrate-to-Ammonia Reduction
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Photocatalytic transformation of nitrate (NO3–) in wastewater into ammonia (NH3) is a challenge in the detoxification and recycling of limited nitrogen resources. In particular, previously reported photocatalysts cannot promote the reaction using water as an electron donor. Herein, we report that copper-doped titanium dioxide (Cu-TiO2) powders, prepared via the sol–gel method and subsequent calcination, promote NO3–-to-NH3 reduction in water. The Cu2+ doping into TiO2 creates a large number of surface oxygen vacancies (OVsurf), which are stable even under aerated conditions. The Ti3+ and Cu2+ atoms adjacent to OVsurf behave as active sites for the NO3–-to-NH3 reduction. Doping with an appropriate amount of Cu2+ and calcination at an appropriate temperature produce the catalysts with a large number of OVsurf, while maintaining a high conductivity, and exhibit a high photocatalytic activity.
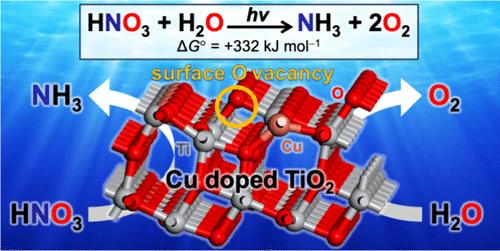
掺杂铜的二氧化钛表面氧空位光催化硝酸还原制氨
废水中硝酸盐(NO3 -)的光催化转化为氨(NH3)是有限氮资源解毒和循环利用的一个挑战。特别是,以前报道的光催化剂不能促进以水作为电子供体的反应。本文报道了通过溶胶-凝胶法制备铜掺杂二氧化钛(Cu-TiO2)粉末,并进行煅烧,促进水中NO3——还原为nh3。Cu2+掺杂到TiO2中会产生大量的表面氧空位(OVsurf),即使在曝气条件下也很稳定。与OVsurf相邻的Ti3+和Cu2+原子是NO3—还原为nh3的活性位点。掺杂适量的Cu2+,在适当的温度下煅烧,得到具有大量OVsurf的催化剂,同时保持高电导率,并表现出较高的光催化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


