Efficient Cytosolic Delivery of Single-Chain Polymeric Artificial Enzymes for Intracellular Catalysis and Chemo-Dynamic Therapy
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Designing artificial enzymes for in vivo catalysis presents a great challenge due to biomacromolecule contamination, poor biodistribution, and insufficient substrate interaction. Herein, we developed single-chain polymeric nanoparticles with Cu/N-heterocyclic carbene active sites (SCNP-Cu) to function as peroxidase mimics for in vivo catalysis and chemo-dynamic therapy (CDT). Compared with the enzyme mimics based on unfolded linear polymer scaffold and multichain cross-linked scaffold, SCNP-Cu exhibits improved tumor accumulation and CDT efficiency both in vitro and in vivo. Protein-like size of the SCNP scaffold promotes passive diffusion, whereas positive surface charge allows its active transcytosis for deep tumor penetration and hence accumulation in the tumor site. The submolecular compartments of the SCNP scaffold effectively protect the active sites from protein bindings, thereby providing a “cleaner” microenvironment for catalysis within a living system. The folded structure of SCNP-Cu facilitates their cytosolic delivery of and free diffusion within cytosol, ensuring efficient contact with endogenous H2O2, in situ generation of toxic hydroxyl radicals (·OH), and effective damage of intracellular targets (i.e., lipids, nucleic acids). This work establishes versatile SCNP-based nanoplatforms for developing artificial enzymes for in vivo catalysis.
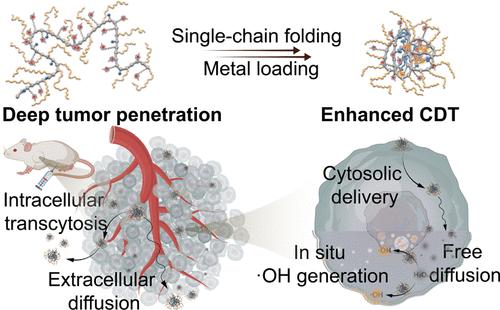
单链聚合人工酶在细胞内催化和化学动力学治疗中的高效细胞质递送
由于生物大分子污染、生物分布差和底物相互作用不足,设计用于体内催化的人工酶面临着巨大的挑战。在此,我们开发了带有Cu/ n -杂环碳烯活性位点(SCNP-Cu)的单链聚合物纳米颗粒,作为过氧化物酶模拟物,用于体内催化和化学动力学治疗(CDT)。与基于未折叠线性聚合物支架和多链交联支架的酶模拟物相比,SCNP-Cu在体外和体内均表现出更好的肿瘤积累和CDT效率。蛋白样大小的SCNP支架促进被动扩散,而表面带正电荷的SCNP支架则允许其主动胞吞作用,从而深入肿瘤渗透并在肿瘤部位积累。SCNP支架的亚分子区室有效地保护活性位点免受蛋白质结合,从而为生命系统内的催化提供“更清洁”的微环境。SCNP-Cu的折叠结构有利于其胞质内递送和胞质内自由扩散,确保与内源性H2O2的有效接触,在原位产生有毒羟基自由基(·OH),并有效破坏细胞内靶标(即脂质,核酸)。这项工作建立了多功能的基于scnp的纳米平台,用于开发用于体内催化的人工酶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


