Photochemical Deracemization of 4,7-Diaza-1-isoindolinones by Unidirectional Hydrogen Atom Shuttling
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
By coupling a photochemical and a thermal step, a single chiral catalyst can establish a photostationary state in which the enantiopure form of a chiral compound is favored over its racemate. Following this strategy, 3-substituted 4,7-diaza-1-isoindolones were successfully deracemized (74–98% yield, 86–99% ee) employing 2.5 mol % of a photocatalyst. Key to the success of the reaction is the fact that a chiral benzophenone recruits selectively one enantiomer of the substrate for a photoinduced hydrogen atom transfer. A combination of computational and experimental studies suggests that the hydrogen atom is shuttled via the oxygen atom of the catalyst to the 4-nitrogen atom of the substrate.
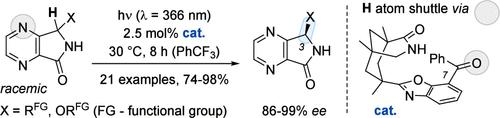
单向氢原子穿梭光化学法对4,7-二氮杂-1-异吲哚酮的脱羧作用
通过光化学和热步骤的耦合,单个手性催化剂可以建立光稳定状态,在这种状态下,手性化合物的对映不纯形式比其外消旋体更有利。在此策略下,使用2.5 mol %的光催化剂,3-取代4,7-二氮杂-1-异吲哚酮成功地离消酰基化(74-98%收率,86-99% ee)。该反应成功的关键是手性二苯酮选择性地招募底物的一个对映体进行光诱导氢原子转移。计算和实验相结合的研究表明,氢原子通过催化剂的氧原子穿梭到底物的4-氮原子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


