Catalytic Enantioselective Synthesis of 1,4-(Hetero) Dicarbonyl Compounds through α-Carbonyl Umpolung
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
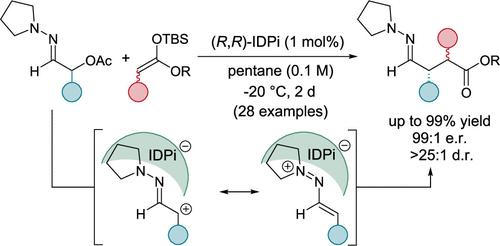
The enantioselective synthesis of 1,4-dicarbonyl compounds continues to pose a significant challenge in organic synthesis, and a catalytic process which generates two adjacent stereogenic centers with full stereochemical control is lacking until now. The 1,4-relationship of the functional groups requires an Umpolung strategy as one of the α-carbonyl positions has to be inverted into an electrophilic center to react with a normal enolate. We report herein the highly enantio- and diastereoselective addition of silyl ketene acetals toward electrophilic 1-azaallyl cations to furnish chiral 4-hydrazonoesters, which are masked 1,4-dicarbonyl compounds. The products carrying up to 2 new stereogenic centers were obtained in excellent yields across a broad substrate scope. As precursors to the 1-azaallyl cations, α-acetoxy hydrazones were employed and ionized with a strongly Lewis acidic, chiral silylium imidodiphosphorimidate (IDPi). The resulting ion pair was characterized with NMR and mass spectroscopy, while DFT calculations provided further insights into the reaction mechanism. In addition, the products were successfully converted into enantiomerically highly enriched b-cyano and b-formyl esters as well as γ-lactams and γ-amino acids, as demonstrated by syntheses of the anticonvulsant agent pregabalin and a brivaracetam precursor.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


