Synergistic Ultrasound-Activable Artificial Enzyme and Precision Gene Therapy to Suppress Redox Homeostasis and Malignant Phenotypes for Controllably Combating Hepatocellular Carcinoma
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Hepatocellular carcinoma (HCC) remains one of the most lethal malignant tumors. Multimodal therapeutics with synergistic effects for treating HCC have attracted increasing attention, for instance, designing biocompatible porphyrin-based nanomedicines for enzyme-mimetic and ultrasound (US)-activable reactive oxygen species (ROS) generation. Despite the promise, the landscape of such advancements remains sparse. Here, we propose the de novo design of a π-conjugated, osmium (Os)-coordinated polyporphyrin (P-Por-Os) nanovesicle to serve as an ultrasound-activable artificial enzyme for synergistic therapies to suppress redox homeostasis and malignant phenotypes for controllably combating HCC. Our findings reveal that the P-Por-Os with US showed superior, multifaceted, and controllable ROS-generating activities. This system not only subverts the redox balance within HCC cells but also achieves precise and controlled tumor ablation at remarkably low concentrations, as evidenced across cellular assays and animal models. In the liver orthotopic model, US not only activates the artificial enzyme to catalyze ROS but also facilitates remote-controlled ablation of HCC through precise US positioning. Moreover, the P-Por-Os + US can assist the precision gene therapy by knocking down the ROS resistance factor, MT2A, and down-regulating its downstream oncogene IGFBP2 to attenuate ROS resistance, proliferation, and migration of HCC efficiently. We suggest that the design of this ultrasound-activable artificial enzyme presents a promising avenue for the engineering of innovative tumoricidal materials, offering a synergistic therapeutic approach with high biosecurity for HCC treatment.
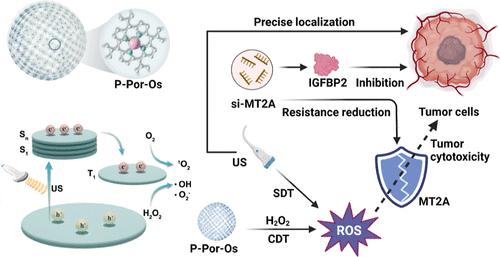
协同超声可激活人工酶和精确基因治疗抑制氧化还原稳态和恶性表型以可控地对抗肝细胞癌
肝细胞癌(HCC)是最致命的恶性肿瘤之一。具有协同作用的多模式治疗方法越来越受到人们的关注,例如设计生物相容性的卟啉纳米药物,用于模拟酶和超声(US)活化活性氧(ROS)的产生。尽管前景看好,但这类进步的前景仍然稀少。在这里,我们提出重新设计π共轭,锇(Os)协调的多卟啉(P-Por-Os)纳米囊泡,作为超声激活的人工酶,用于协同治疗,抑制氧化还原稳态和恶性表型,以可控地对抗HCC。我们的研究结果表明,具有US的P-Por-Os具有优越的,多方面的,可控的ros生成活性。该系统不仅破坏HCC细胞内的氧化还原平衡,而且可以在非常低的浓度下实现精确和可控的肿瘤消融,这在细胞实验和动物模型中得到了证明。在肝脏原位模型中,US不仅激活人工酶催化ROS,还可以通过精确的US定位实现对HCC的遥控消融。此外,P-Por-Os + US可以通过下调ROS抵抗因子MT2A,并下调其下游致癌基因IGFBP2,有效地减弱HCC的ROS抵抗、增殖和迁移,从而辅助精准基因治疗。我们认为,这种超声激活人工酶的设计为创新的杀瘤材料的工程设计提供了一条有前途的途径,为HCC治疗提供了一种具有高生物安全性的协同治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


