Copper-Catalyzed Asymmetric Nucleophilic Opening of 1,1,2,2-Tetrasubstituted Donor–Acceptor Cyclopropanes for the Synthesis of α-Tertiary Amines
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
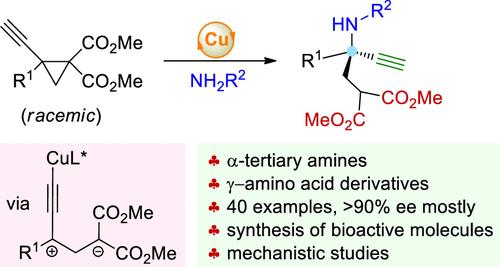
Catalytic asymmetric transformation of donor–acceptor cyclopropanes (DACs) has been proven to be a highly valuable and robust strategy to construct diverse types of enantioenriched molecules. However, the use of 1,1,2,2-tetrasubstituted DACs to form products bearing quaternary stereocenters remains a long-term unsolved challenge. Here, we report the copper-catalyzed asymmetric aminative ring opening of tetrasubstituted alkynyl DACs that delivers a myriad of α-tertiary amines with high levels of enantioselectivities. The alkyne, amine, and ester moieties within the products enable diverse further applications, including the asymmetric synthesis of bioactive molecules. Mechanistic studies indicate that the zwitterionic intermediate bearing a copper-acetylide unit plays a key role in the process, which represents a new mode for achieving catalytic asymmetric transformation of DACs.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


