Synthesis of Two-Dimensional High-Entropy Transition Metal Dichalcogenide Single Crystals
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Two-dimensional (2D) high-entropy transition metal dichalcogenides (HETMDs) have gained significant interest due to their structural properties and correlated possibilities for high-end devices. However, the controlled synthesis of 2D HETMDs presents substantial challenges owing to the distinction in the inherent characteristics among diverse metal elements in the synthesis, such as saturated vapor pressure of precursors and formation energy of products. Here, we present the synthesis of a 2D HETMD single crystal with 0.92 nm thickness through a liquid-phase reaction system, where the metal elements are fed uniformly and simultaneously. The rapid codeposition of different precursors facilitates the formation of high-entropy products, thereby preventing phase separation. The method can be expanded to produce a variety of 2D HETMDs, such as quinary (MoNbTaV)S2, hexahydroxy (MoWNbTaV)S2, and multichalcogenide (MoWNb)SSe. The as-prepared 2D HETMD is an excellent catalyst for the hydrogen evolution reaction (HER), demonstrating the overpotential of 84 mV at 10 mA cm–2 of an individual crystal, which is much better than that of pristine MoS2 (260 mV at 10 mA cm–2). The strategy offers the flexibility to artificially design the element selectivity and properties of HETMD single crystals in the 2D limit, enabling applications across a wide range of advanced fields.
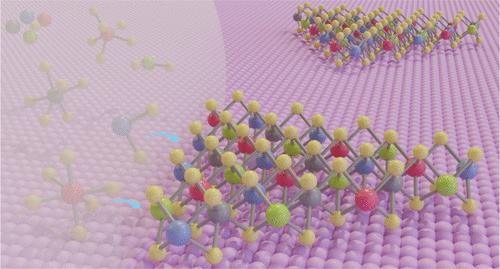
二维高熵过渡金属二硫族化物单晶的合成
二维(2D)高熵过渡金属二硫族化合物(HETMDs)由于其结构特性和高端器件的相关可能性而获得了极大的兴趣。然而,由于合成过程中不同金属元素的固有特性(如前驱体的饱和蒸汽压和产物的形成能)存在差异,二维HETMDs的受控合成面临着很大的挑战。本文采用液相反应体系,在均匀、同步加料的条件下,合成了厚度为0.92 nm的二维HETMD单晶。不同前驱体的快速共沉积促进了高熵产物的形成,从而防止了相分离。该方法可以扩展到多种二维HETMDs,如五元(MoNbTaV)S2,六羟基(MoWNbTaV)S2和多硫系(MoWNb)SSe。制备的二维HETMD是一种极好的析氢反应催化剂,单晶在10 mA cm-2时的过电位为84 mV,远优于原始MoS2 (10 mA cm-2时的过电位为260 mV)。该策略提供了在2D限制下人为设计HETMD单晶的元素选择性和性能的灵活性,使其能够在广泛的先进领域中得到应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


