Selective Reduction of Esters to Access Aldehydes Using Fiddler Crab-Type Boranes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
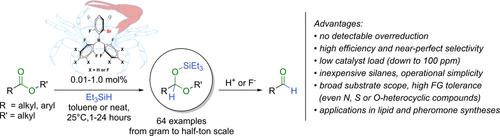
The partial reduction of esters to aldehydes is a fundamentally important transformation for the synthesis of numerous fine chemicals and consumer goods. However, despite the many efforts, limitations have persisted, such as competing overreduction, low reproducibility, use of exigent reaction conditions and hazardous chemicals. Here, we report a novel catalyst family with a unique steric design which promotes the catalytic partial reduction of esters with unprecedented, near-perfect selectivity and efficiency. This metal-free catalytic method is ready to be placed at the disposal of chemists to provide valuable aldehyde intermediates and products and shows promise for streamlining synthetic methods in academic and industrial settings.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


