Consecutive C–C Coupling of CH4 and CO2 Mediated by Heteronuclear Metal Cations CuTa+
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The conversion of methane and carbon dioxide to form C2 products is of great interest but presents a long-standing grand challenge due to the significant obstacle of activating the inert C–H and C═O bonds as well as forming the C–C bonds. Herein, the consecutive C–C coupling of CH4 and CO2 was realized by using heteronuclear metal cations CuTa+, and the desorption of H2C═C═O molecules was evidenced by state-of-the-art mass spectrometry. The CuTa+ reaction system is significantly different from the homonuclear metal systems of Cu2+ and Ta2+. On the basis of density functional theory calculations, we identified that Cu can modulate the charge distribution and reduce the energy difference of crucial orbitals for the C–C coupling of CH2 and CO units that are from the activation of CH4 and CO2, respectively. The crucial role of the Cu atom is of substantial importance to understand the process of the C–C coupling reaction in Cu-based heterogeneous catalytic systems. This study not only provides a promising paradigm for the design of non-noble metal species in direct conversion of CH4 and CO2 under mild conditions but also reveals a new molecular-level mechanism of consecutive C–C coupling for the production of H2C═C═O, a crucial intermediate during carbonylation reactions.
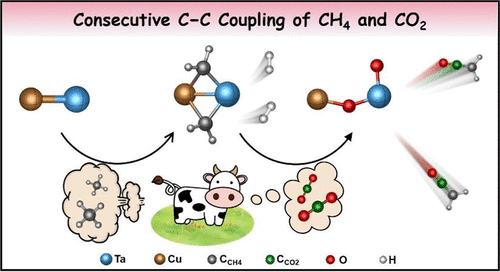
异核金属阳离子CuTa+介导的CH4与CO2连续C-C偶联
甲烷和二氧化碳的转化形成C2产物是非常有趣的,但由于激活惰性的C - h和C = O键以及形成C - C键的显著障碍,提出了一个长期存在的巨大挑战。本文利用异核金属阳离子CuTa+实现了CH4和CO2的连续C - C偶联,并通过最先进的质谱技术证明了H2C = C = O分子的解吸。CuTa+反应体系与Cu2+和Ta2+的同核金属体系有明显不同。基于密度功能理论计算,我们发现Cu可以调节CH2和CO单元C-C耦合的电荷分布,减小CH4和CO2分别激活的关键轨道的能量差。Cu原子的关键作用对于理解Cu基非均相催化体系中C-C偶联反应的过程具有重要意义。这项研究不仅为设计在温和条件下直接转化CH4和CO2的非贵金属物种提供了一个有希望的范例,而且揭示了一种新的分子水平的连续C - C偶联机制,用于生产羰基化反应中的关键中间体H2C = C = O。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


