Targeting P4HA1 promotes CD8+ T cell progenitor expansion toward immune memory and systemic anti-tumor immunity
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Successful immunotherapy relies on both intratumoral and systemic immunity, which is yet to be achieved for most patients with cancer. Here, we identify P4HA1, encoding prolyl 4-hydroxylase 1, as a crucial regulator of CD8+ T cell differentiation strongly upregulated in tumor-draining lymph nodes (TDLNs) and hypoxic tumor microenvironment. P4HA1 accumulates in mitochondria, disrupting the tricarboxylic acid (TCA) cycle through aberrant α-ketoglutarate and succinate metabolism, promoting mitochondria unfitness and exhaustion while suppressing progenitor expansion. Targeting P4HA1 enhances both adoptive and endogenous TCF1+ CD8+ T progenitor expansion while mitigating the development of exhaustion in the tumor, TDLN, and blood, enabling a notable and durable systemic anti-cancer immunity. We propose that P4HA1 induction in CD8+ T cells in cancer orchestrates an immune-escape program, offering a T cell-directed target for system immunotherapy in solid tumors.
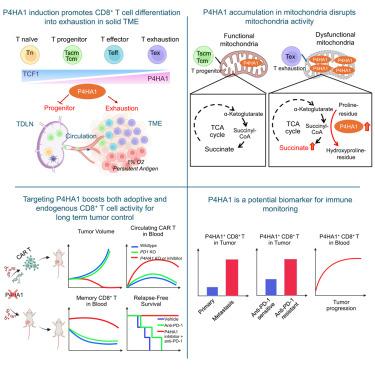
靶向P4HA1促进CD8+ T细胞祖细胞向免疫记忆和全身抗肿瘤免疫的扩展
成功的免疫治疗依赖于肿瘤内和全身免疫,这对于大多数癌症患者来说尚未实现。在这里,我们发现编码脯氨酸4-羟化酶1的P4HA1是CD8+ T细胞分化的关键调节因子,在肿瘤引流淋巴结(tdln)和缺氧肿瘤微环境中被强烈上调。P4HA1在线粒体中积累,通过α-酮戊二酸和琥珀酸代谢异常破坏三羧酸(TCA)循环,促进线粒体不适应和衰竭,同时抑制祖细胞扩张。靶向P4HA1可增强过继性和内源性TCF1+ CD8+ T祖细胞的扩增,同时减轻肿瘤、TDLN和血液中的衰竭发展,从而实现显著且持久的全身抗癌免疫。我们提出P4HA1在癌症中诱导CD8+ T细胞协调免疫逃逸程序,为实体瘤的系统免疫治疗提供T细胞导向的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


