Diazocines as Guests of Cucurbituril Macrocycles: Light-Responsive Binding and Supramolecular Catalysis of Thermal Isomerization
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
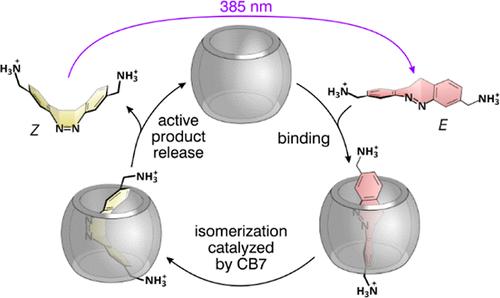
The photoswitching of supramolecular host–guest complexes is the basis of numerous molecularly controlled macroscopic functions, such as sol–gel transition, photopharmacology, the active transport of ions or molecules, light-powered molecular machines, and much more. The most commonly used systems employ photoactive azobenzene guests and synthetic host molecules, which bind as the stable E isomers and dissociate as the Z forms after exposure to UV light. We present a new, extraordinarily efficient cucurbit[7]uril (CB7)/diazocine host/guest complex with inverted stability that self-assembles under UV irradiation and dissociates in the dark. The association constants of the Z and E isomers in water differ by more than 104-fold. We also show that the thermally activated E → Z isomerization is significantly accelerated by CB7, which is a rare case of enzyme-like catalysis by transition state stabilization without product inhibition. In contrast to CB7, cucurbit[8]uril (CB8) binds both isomers with high affinity, showing good selectivity (∼1000-fold) toward the Z isomer. Notably, this isomer preferentially binds CB8 relative to CB7 by a factor greater than 1 × 106. We also use the system to introduce a supramolecular photoacid that builds on the increased basicity of a guest bound to CB7 and on the extremely high affinity of the E isomer, which is utilized to displace the acid from CB7, thereby switching the pH of the solution.
重氮嘧啶作为瓜脲类大环的客体:光响应结合和热异构化的超分子催化
超分子主客体复合物的光开关是许多分子控制宏观功能的基础,如溶胶-凝胶转变、光药理学、离子或分子的主动运输、光动力分子机器等等。最常用的系统采用光活性偶氮苯客体和合成宿主分子,它们结合为稳定的E异构体,并在暴露于紫外线后作为Z形式解离。我们提出了一种新的,非常高效的葫芦[7]uril (CB7)/重氮嘧啶主/客体配合物,具有倒置稳定性,在紫外线照射下自组装,在黑暗中解离。Z和E异构体在水中的缔合常数相差超过104倍。我们还发现,CB7显著加速了热激活的E→Z异构化,这是一种罕见的通过过渡态稳定而没有产物抑制的类酶催化。与CB7相比,葫芦[8]uril (CB8)与两种异构体具有高亲和力,对Z异构体具有良好的选择性(约1000倍)。值得注意的是,相对于CB7,该异构体优先结合CB8的因子大于1 × 106。我们还使用该系统引入了一种超分子光酸,该光酸建立在与CB7结合的客体的碱性增加和E异构体的极高亲和力的基础上,该异构体被用来取代CB7中的酸,从而改变溶液的pH值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


