Efficacy and Safety of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting Transforming Growth Factor-β and Programmed Death-Ligand 1, Plus Chemotherapy in Patients With Stage IV NSCLC
IF 3.5
Q2 ONCOLOGY
引用次数: 0
Abstract
Introduction
In a phase 1 study, bintrafusp alfa was found to have an encouraging clinical activity in patients with previously treated advanced NSCLC. This study evaluated the safety and efficacy of bintrafusp alfa with chemotherapy in patients with stage IV NSCLC regardless of the programmed death-ligand 1 (PD-L1) expression status.
Methods
In this open-label, phase 1b/2 study (NCT03840915), eligible patients were assigned to one of four cohorts. Patients with previously untreated metastatic NSCLC (cohorts A, B, and C) received bintrafusp alfa with chemotherapy as first-line treatment, whereas patients whose disease progressed on previous treatment with programmed cell death protein 1 or PD-L1 inhibitors (cohort D) received bintrafusp alfa with chemotherapy as second-line treatment. The primary objective of this study was to evaluate the safety and tolerability of bintrafusp alfa with chemotherapy.
Results
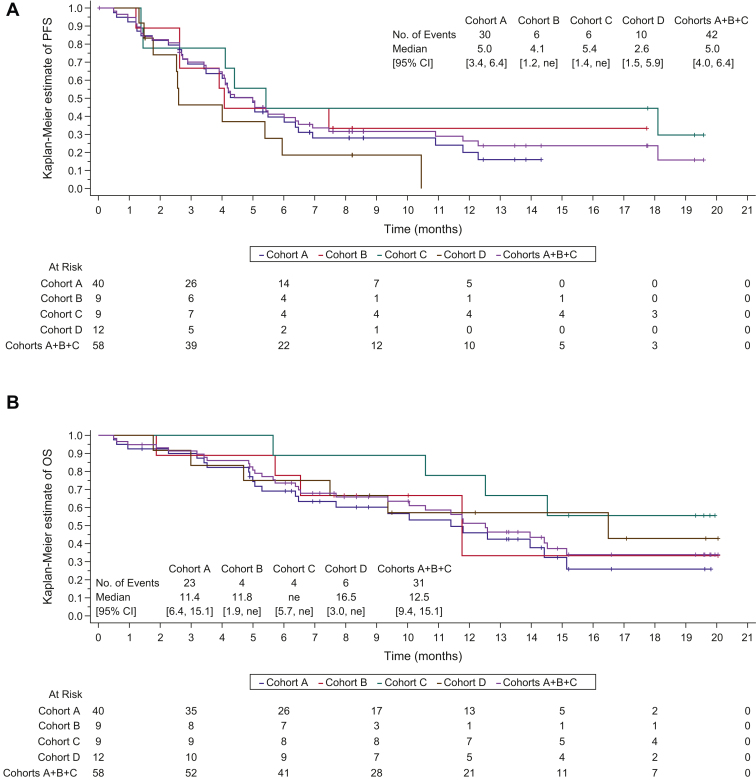
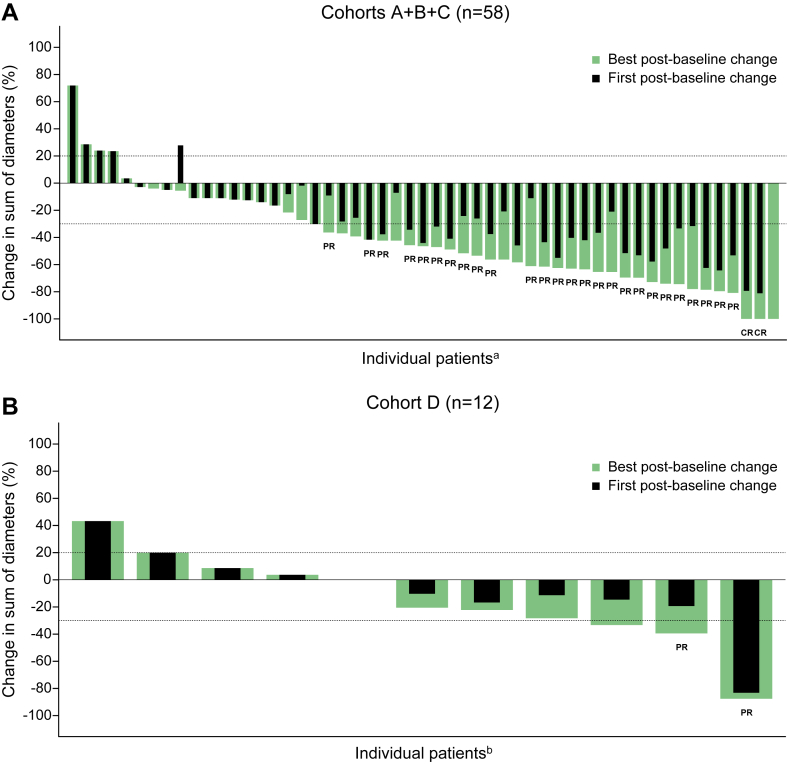
Four serious and one nonserious treatment-emergent adverse events were considered dose-limiting toxicities, none of which were assessed as related to bintrafusp alfa by the investigator. Any-grade bintrafusp alfa-related adverse events occurred in 20.7% of patients in cohorts A+B+C and in 16.7% of patients in cohort D. Keratoacanthoma was the most common transforming growth factor-β inhibition-mediated skin lesion (cohorts A+B+C: 12.1% and cohort D: 8.3%). In cohorts A+B+C, the overall response rate was 48.3%, and in patients with PD-L1 tumor proportion score of more than or equal to 50.0%, it was 71.4%. On the basis of an interim analysis, the data were considered mature, and no further analysis has been planned.
Conclusion
Bintrafusp alfa with chemotherapy was found to have a manageable safety profile and encouraging clinical activity in patients with stage IV NSCLC.
靶向转化生长因子-β和程序性死亡配体1的双功能融合蛋白Bintrafusp Alfa加化疗在IV期NSCLC患者中的疗效和安全性
在一项i期研究中,发现bintrafusp在既往治疗过的晚期NSCLC患者中具有令人鼓舞的临床活性。本研究评估了bintrafusp α在IV期NSCLC患者化疗中的安全性和有效性,无论其程序性死亡配体1 (PD-L1)表达状态如何。方法:在这项开放标签的1b/2期研究(NCT03840915)中,符合条件的患者被分配到四个队列中的一个。先前未接受治疗的转移性NSCLC患者(队列A、B和C)接受bintrafusp alfa联合化疗作为一线治疗,而先前接受程序性细胞死亡蛋白1或PD-L1抑制剂治疗的疾病进展患者(队列D)接受bintrafusp alfa联合化疗作为二线治疗。本研究的主要目的是评估bintrafusp与化疗的安全性和耐受性。结果:4个严重和1个非严重的治疗不良事件被认为是剂量限制性毒性,没有一个被研究者评估为与bintrafusp有关。A+B+C组中20.7%的患者发生了任何级别的bintrafusp α相关不良事件,D组中16.7%的患者发生了任何级别的bintrafusp α相关不良事件。角棘瘤是最常见的转化生长因子-β抑制介导的皮肤病变(A+B+C组:12.1%,D组:8.3%)。在A+B+C队列中,总有效率为48.3%,在PD-L1肿瘤比例评分大于等于50.0%的患者中,总有效率为71.4%。根据一项临时分析,这些数据被认为是成熟的,没有计划进一步的分析。结论:Bintrafusp联合化疗在IV期NSCLC患者中具有可控的安全性和促进的临床活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


