Chiral Phosphoric Acid-Catalyzed Enantioselective Synthesis of 2,2-Disubstituted 2,3-Dihydro-4-quinolones from Isatins and 2′-Aminoacetophenones
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we present the enantioselective synthesis of 2,3-dihydro-4-quinolones bearing chiral tetrasubstituted carbons from isatins and 2′-aminoacetophenones. The transformation is mediated by a chiral phosphoric acid catalyst and proceeds via an in situ generated ketimine and subsequent enantioselective intramolecular cyclization. The methodology features a broad scope and functional group tolerance with yields and enantioselectivities of up to 99% and 98% ee. Detailed density functional theory (DFT) calculations support the proposed reaction mechanism and the origin of asymmetric induction.
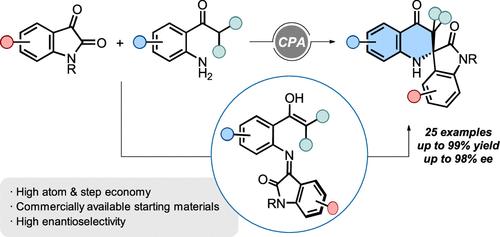
手性磷酸催化2′-氨基苯乙酮和Isatins合成2,2-二取代2,3-二氢-4-喹诺酮类药物
本研究以鸢尾素和2′-氨基苯乙酮为原料,对映选择性地合成了手性四取代碳的2,3-二氢-4-喹诺酮。该转化由手性磷酸催化剂介导,并通过原位生成的氯胺酮和随后的对映选择性分子内环化进行。该方法具有广泛的适用范围和功能基团耐受性,产率和对映选择性分别高达99%和98% ee。详细的密度泛函理论(DFT)计算支持所提出的反应机理和不对称感应的起源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


