Merging and Clipping Nets for the Synthesis of Three- and Two-Merged Net Metal–Organic Frameworks
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
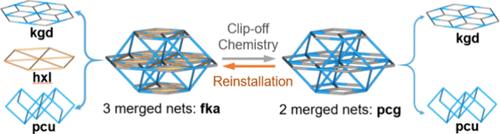
Herein, we report how merging and clipping nets in metal–organic frameworks (MOFs) can be controlled in a single-crystal-to-single-crystal fashion using three different approaches─the merged net, clip-off chemistry, and linker reinstallation─to design and synthesize three- and two-merged net MOFs. Initially, we show the formation of three isoreticular three-merged net MOFs by linking a trimeric Sc3+ cluster, Sc3(μ3-Ο)(−COO)6, with ditopic zigzag and tritopic linkers. The resulting MOFs exhibit three-merged edge-transitive nets─kgd + hxl + pcu─for the first time. Then, using these three-merged net MOFs as precursors, we selectively remove one of these subnets, the hxl net, via clip-off chemistry to form two-merged net MOFs. This process involves the cleavage of olefinic groups via ozonolysis, providing the resulting two-merged net MOFs with free carboxylic acid groups that can be used to tune their sorption properties such as the removal of cationic organic pollutants. Finally, we use the linker reinstallation approach to convert the two-merged net MOFs back to the three-merged net MOFs. This approach allows for the postsynthetic addition of the previously removed hxl merged net, enabling recovery of the initial three-merged net MOFs or synthesis of new ones using novel ditopic zigzag linkers.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


