In-Cage Recombination Facilitates the Enantioselective Organocatalytic [1,2]-Rearrangement of Allylic Ammonium Ylides
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
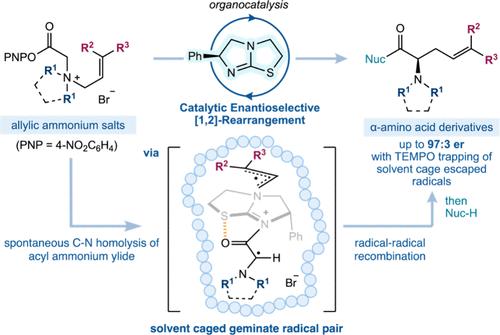
The [1,2]-rearrangement of allylic ammonium ylides is traditionally observed as a competitive minor pathway alongside the thermally allowed [2,3]-sigmatropic rearrangement. Concerted [1,2]-rearrangements are formally forbidden, with these processes believed to proceed through homolytic C–N bond fission of the ylide, followed by radical–radical recombination. The challenges associated with developing a catalytic enantioselective [1,2]-rearrangement of allylic ammonium ylides therefore lie in biasing the reaction pathway to favor the [1,2]-reaction product, alongside controlling a stereoselective radical–radical recombination event. Herein, a Lewis basic chiral isothiourea facilitates catalytic [1,2]-rearrangement of prochiral aryl ester ammonium salts to generate unnatural α-amino acid derivatives with up to complete selectivity over the [2,3]-rearrangement and with good to excellent enantiocontrol. Key factors in favoring the [1,2]-rearrangement include exploitation of disubstituted terminal allylic substituents, cyclic N-substituted ammonium salts, and elevated reaction temperatures. Mechanistic studies involving 13C-labeling and crossover reactions, combined with radical trapping experiments and observed changes in product enantioselectivity, are consistent with a radical solvent cage effect, with maximum product enantioselectivity observed through promotion of “in-cage” radical–radical recombination. Computational analysis indicates that the distribution between [1,2]- and [2,3]-rearrangement products arises predominantly from C–N bond homolysis of an intermediate ammonium ylide, followed by recombination of the α-amino radical at either the primary or tertiary site of an intermediate allylic radical. Electrostatic interactions involving the bromide counterion control the facial selectivity of the [1,2]- and [2,3]-rearrangements, while the sterically hindered tertiary position of the allylic substituent disfavors the formation of the [2,3]-product. These results will impact further investigations and understanding of enantioselective radical–radical reactions.
笼内重组促进了烯丙基酰化铵的对映选择性有机催化[1,2]重排
传统上,烯丙基铵酰化物的[1,2]-重排被认为是与热允许的[2,3]-异位重排并列的竞争性次要途径。一致的[1,2]重排在形式上是被禁止的,这些过程被认为是通过ylide的均溶C-N键裂变进行的,然后是自由基-自由基重组。因此,开发烯丙基酰化铵催化对映选择性[1,2]重排的挑战在于使反应途径偏向于[1,2]-反应产物,同时控制立体选择性自由基-自由基重组事件。本文中,路易斯碱性手性异硫脲促进了前手性芳基酯铵盐的[1,2]重排,生成非天然α-氨基酸衍生物,对[2,3]重排具有完全的选择性,并具有良好到优异的对映控制。有利于[1,2]重排的关键因素包括利用末端二取代的烯丙基取代基、环n取代铵盐和升高的反应温度。通过13c标记和交叉反应的机理研究,结合自由基捕获实验和观察到的产物对映体选择性的变化,发现自由基溶剂笼效应是一致的,通过促进“笼内”自由基-自由基的重组,可以观察到最大的产物对映体选择性。计算分析表明,[1,2]-和[2,3]-重排产物之间的分布主要是由中间酰化铵的C-N键均解引起的,其次是中间烯丙基自由基的一级或三级位点上α-氨基自由基的重组。涉及溴离子的静电相互作用控制了[1,2]-和[2,3]-重排的表面选择性,而烯丙基取代基的空间阻碍三级位置不利于[2,3]-产物的形成。这些结果将影响对映选择性自由基-自由基反应的进一步研究和理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


