The Role of Nonequilibrium Solvent Effects in Enhancing Direct CO2 Capture at the Air–Aqueous Amino Acid Interface
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Direct air capture (DAC) technologies are limited by the poor understanding of the dynamic role of interfaces in modulating the chemisorption of CO2 from air into solutions. While the reactivity of aqueous amine-based solvents in the bulk environment is strongly inhibited by nonequilibrium solvent effects, promoting DAC at interfaces posits a possibility to reduce the coupling with the solvent and significantly accelerate DAC. Building on an experimentally proven concept to bring an anionic glycine absorbent to the interface through ion-pairing interactions with a positively charged surfactant, we establish the fundamental time scales for key elementary steps involved in DAC with rate theory and enhanced-sampling ab initio molecular dynamics simulations. We elucidate the mechanism by which water influences the free energy barriers and dynamical crossing-recrossing of those barriers, affecting the reaction rates. We find that water reorganizes to partially dehydrate [-NH2], facilitating SN2-based CO2 conversion to a zwitterion, which then releases a proton via overhydration of [-NH2]. The low-density interfacial water favors dehydration over overhydration, leading to a comparatively higher barrier (slower kinetics) for proton release. The barrier-recrossing events neutralize this effect, letting both steps occur at the same time scale (sub-microseconds) and making the overall DAC process faster at the interface than in the bulk water. Such an understanding of environment-sensitive solvent effects on the reaction kinetics will help design tailored interfaces for enhanced CO2 capture kinetics via control of solvation and ion paring.
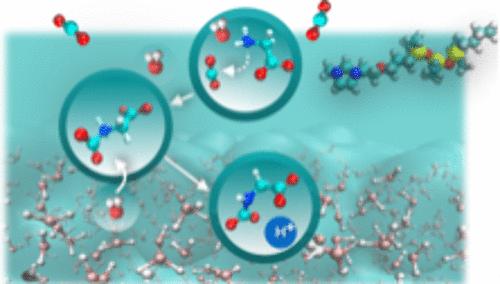
非平衡溶剂效应在促进空气-水氨基酸界面CO2直接捕获中的作用
直接空气捕获(DAC)技术由于对界面在调节二氧化碳从空气中化学吸附到溶液中的动态作用的理解不足而受到限制。虽然胺基溶剂在体环境中的反应性受到非平衡溶剂效应的强烈抑制,但在界面处促进DAC有可能减少与溶剂的耦合并显着加速DAC。通过与带正电荷的表面活性剂的离子配对相互作用,将阴离子甘氨酸吸收剂带到界面上,我们建立了一个实验证明的概念,通过速率理论和增强采样从头算分子动力学模拟,建立了DAC关键基本步骤的基本时间尺度。我们阐明了水影响自由能垒和这些垒的动态交叉-再交叉,从而影响反应速率的机理。我们发现水重组使[-NH2]部分脱水,促进基于sn2的CO2转化为两性离子,然后通过[-NH2]的过度水化释放一个质子。低密度的界面水有利于脱水而不是过度水化,导致质子释放的相对较高的屏障(较慢的动力学)。跨越屏障事件抵消了这种影响,使这两个步骤在同一时间尺度(亚微秒)发生,并使整个DAC过程在界面上比在大量水中更快。这种对环境敏感溶剂对反应动力学的影响的理解将有助于设计定制的界面,通过控制溶剂化和离子配对来增强二氧化碳捕获动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


