Highly Efficient Transpeptidase-Catalyzed Isopeptide Ligation
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Transpeptidases are specialized enzymes that have evolved for site-selective modification of peptides and proteins at their backbone termini. Approaches for adapting transpeptidases to catalyze side chain modifications are substantially more restricted, and typically rely on large recognition tags or require specific reaction conditions that are not easily compatible with broader applications. Here we show that the engineered asparaginyl ligase OaAEP1 catalyzes direct isopeptide ligation by accepting an internal 2,3-diaminopropionic acid (Dap) residue adjacent to Leu, a motif that mimics the canonical N-terminal Gly-Leu substrate. These reactions proceed efficiently at near-neutral pH without any required additives, enabling straightforward formation of diverse isopeptide-linked products under simple reaction conditions. We demonstrate that OaAEP1-catalyzed isopeptide ligation can be utilized for site-selective side chain labeling at an introduced Dap residue with minimal off-target labeling of Lys residues. Additionally, we generate engineered peptide topologies via intramolecular side chain-to-tail cross-links and produce direct protein–cyclic peptide fusions via efficient intermolecular ligation. We also show that OaAEP1-catalyzed isopeptide ligation extends to d-peptide acceptors containing a retro-inverso d-Leu-d-Dap motif. This capability further expands the range and complexity of isopeptide-linked products that can be accessed with OaAEP1, which we exemplify by forming a hybrid d-/l- bicyclic peptide topology where both termini are linked to internal side chains.
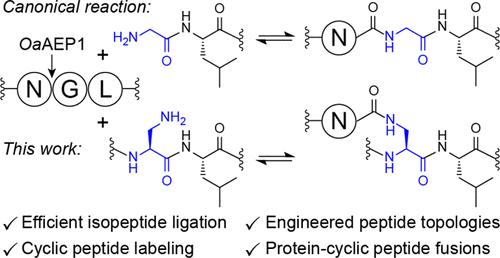
高效转肽酶催化的异肽连接
转肽酶是一种特殊的酶,它可以在肽和蛋白质的主干末端进行位点选择性修饰。适应转肽酶催化侧链修饰的方法实质上更受限制,并且通常依赖于大型识别标签或需要特定的反应条件,这些条件不容易与更广泛的应用相兼容。本研究表明,工程天冬酰胺酰连接酶OaAEP1通过接受Leu附近的2,3-二氨基丙酸(Dap)残基来催化直接异肽连接,这是一个模仿典型n端Gly-Leu底物的基序。这些反应在接近中性的pH值下有效进行,不需要任何添加剂,可以在简单的反应条件下直接形成各种异肽连接产物。我们证明,oaaep1催化的异肽连接可以用于在引入的Dap残基上进行位点选择性侧链标记,并且对Lys残基进行最小的脱靶标记。此外,我们通过分子内侧链到尾部交联产生工程肽拓扑结构,并通过有效的分子间连接产生直接的蛋白环肽融合。我们还发现oaaep1催化的异肽连接延伸到含有逆转录逆d-Leu-d-Dap基序的d肽受体。这种能力进一步扩大了OaAEP1可访问的异肽连接产物的范围和复杂性,我们通过形成混合d-/l-双环肽拓扑来举例说明,其中两个末端都连接到内部侧链。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


