Fluoroform (CHF3) Production from CF3CHO Photolysis and Implications for the Decomposition of Hydrofluoroolefins and Hydrochlorofluoroolefins in the Atmosphere
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Hydrofluoroolefins (HFOs) and hydrochlorofluoroolefins (HCFOs) are the leading synthetic replacements for compounds successively banned by the Montreal Protocol and amendments. HFOs and HCFOs readily decompose in the atmosphere to form fluorinated carbonyls, including CF3CHO in yields of up to 100%, which are then photolyzed. A long-standing issue, critical for the transition to safe industrial gases, is whether atmospheric decomposition of CF3CHO yields any quantity of CHF3 (HFC-23), which is one of the most environmentally hazardous greenhouse gases. This comprehensive experimental investigation employs purpose-built photoionization mass spectrometry, Fourier-transform infrared, and microwave spectroscopy techniques and confirms production of CHF3 following excitation at a tropospherically relevant wavelength (λ = 308 nm) and under atmospheric pressure conditions. Pressure-dependent CHF3 quantum (Φ) and molar (Y) yields are reported from Φ = Y = 0.16 ± 0.03 under collision-free conditions to Φ = (2.3 ± 0.3) × 10–4, Y = (1.17 ± 0.27) × 10–3 at 1 bar N2.
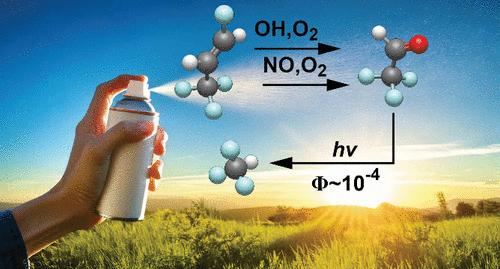
CF3CHO光解产氟仿(CHF3)及其对大气中氢氟烯烃和氢氯氟烯烃分解的影响
氢氟烯烃(hfo)和氢氯氟烯烃(hcfo)是《蒙特利尔议定书》及其修正案相继禁止的化合物的主要合成替代品。hfo和hcfo很容易在大气中分解,形成含氟羰基,包括CF3CHO,其产量高达100%,然后被光解。一个长期存在的问题,对向安全工业气体过渡至关重要,那就是CF3CHO在大气中的分解是否会产生一定数量的CHF3 (HFC-23),这是对环境危害最大的温室气体之一。这项综合实验研究采用了专门建立的光电离质谱、傅里叶变换红外和微波光谱技术,并证实了在对流层相关波长(λ = 308 nm)和大气压条件下激发后CHF3的产生。压力依赖性CHF3量子(Φ)和摩尔(Y)产率从无碰撞条件下Φ = Y = 0.16±0.03到1bar N2条件下Φ =(2.3±0.3)× 10-4, Y =(1.17±0.27)× 10-3。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


