Electrophilic Substitution as a Mechanism for Ligand Exchange Reactions on Silver Monolayer-Protected Clusters
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
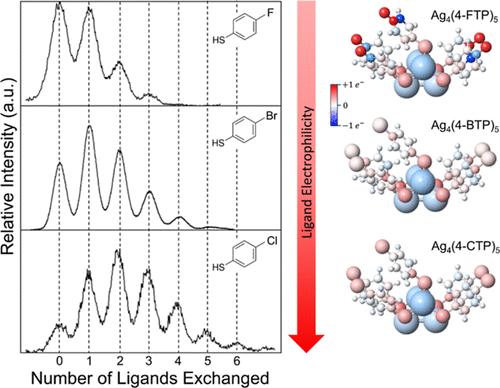
Chemical research on noble metal monolayer-protected clusters (MPCs) has typically focused on discovering new sizes and compositions. However, the discovery of the fundamental chemical principles that govern MPC reactions, which are required for the rational chemical transformation of MPCs, has received significantly less attention. Here, we study in detail the electronic nature of the mechanism of postsynthetic ligand exchange reactions using M4Ag44(p-MBA)30 as a model system, where M is a monocationic counterion and p-MBA is para-mercaptobenzoic acid. The systematic exchange of aryl thiol ligands, with different electron-withdrawing and -donating substituents in different ring positions, was studied in detail by electrospray-ionization mass spectrometry measurements of equilibrium product distributions and density functional theory (DFT) calculations of fully ligand-exchanged clusters. We found that (i) these ligand exchange reactions are driven by the relative electrophilicity of the incoming ligands, (ii) the electrophilic driving force was stronger than modest steric effects, and (iii) site-specific substitution due to ligand shell bonding geometries was not observed, revealing certain limitations of rational synthesis. DFT calculations uncovered the complexity of the charge distributions on the clusters, contrasting with currently used simple heuristic models of charge withdrawal and calling attention to the subtlety and intricacy of the effects of charge redistribution upon substitution. By carefully and systematically establishing such basic chemical principles to build a catalog of MPC reactions, in analogy to organic chemistry, the rational synthesis of new MPC materials using MPCs as reagents in transformation reactions could become possible.
亲电取代:银单层保护簇上配体交换反应的机制
对贵金属单层保护簇(MPCs)的化学研究通常集中在发现新的尺寸和成分上。然而,控制MPC反应的基本化学原理的发现,这是MPC合理化学转化所必需的,受到的关注却少得多。本文以M4Ag44(p-MBA)30为模型体系,其中M为单阳离子反离子,p-MBA为对巯基苯甲酸,详细研究了合成后配体交换反应机理的电子性质。通过电喷雾电离质谱测量平衡产物分布和密度泛函理论(DFT)计算,详细研究了芳基硫醇配体在不同环位上具有不同吸电子和供电子取代基的系统交换。我们发现:(i)这些配体交换反应是由进入配体的相对亲电性驱动的,(ii)亲电性驱动力比适度的空间效应强,(iii)由于配体壳键的几何形状而导致的位点特异性取代没有被观察到,揭示了合理合成的某些局限性。DFT计算揭示了簇上电荷分布的复杂性,与目前使用的简单的电荷提取启发式模型形成对比,并引起人们对电荷再分配对取代影响的微妙和复杂性的关注。通过认真、系统地建立这些基本的化学原理,建立MPC反应的目录,就可以像有机化学一样,利用MPC作为转化反应的试剂,合理地合成新的MPC材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


