Enhanced Structural and Electrochemical Stability of Li and Mg Co-Doped LiMn2O4 Cathodes for Li-Ion Batteries with a Mg Source from Bischofite
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this work, Li1+xMgyMn2–x–yO4 spinel octahedral nanoparticles doped with Li and Mg (x = 0.03, y = 0.00, 0.02, 0.05, and 0.10) were synthesized by an ultrasound-assisted Pechini-type sol–gel process. High-purity Mg(OH)2, obtained from bischofite (MgCl2·6H2O), an industrial waste produced during the industrial lithium extraction process, was used as a new source of magnesium for this purpose. Electrochemical measurements were carried out in coin semicells. As the Mg doping concentration increases, the insertion/extraction mechanism of Li ions changes from a two-phase reaction to a single-phase process in a solid solution. The discharge capacities of the cathode materials increase until reaching a maximum value of 120.2 mAh g–1 for y = 0.05 and finally decrease for y = 0.10. The retention capacity exhibited the same behavior after 100 cycles at a C/3 rate and 18 °C, with the optimum (y = 0.05, 94.0%). The retention capacity dropped by 14% at 50 °C. The optimum was successfully applied in pouch cells at 50 cycles with similar and stable electrochemical behavior. Therefore, both Mg(OH)2 and Li2CO3 obtained from Salar de Atacama are sources of Mg and Li, potential candidates in the development of cathode materials with high-rate capability and long cycling stability for lithium-ion batteries.
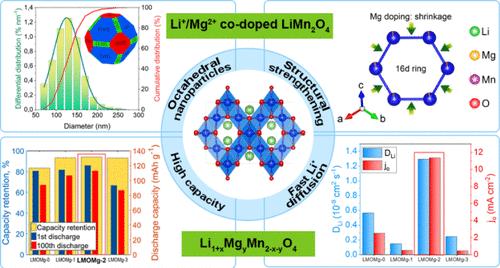
铋辉石镁源锂离子电池中Li和Mg共掺杂LiMn2O4阴极的结构和电化学稳定性
本文采用超声辅助pechini型溶胶-凝胶法制备了Li + xMgyMn2-x-yO4尖晶石八面体纳米粒子(x = 0.03, y = 0.00, 0.02, 0.05, 0.10)。利用工业锂提取过程中产生的工业废渣菱白石(MgCl2·6H2O)获得高纯镁(OH)2作为镁的新来源。电化学测量是在硬币半电池中进行的。随着Mg掺杂浓度的增加,Li离子在固溶体中的插入/萃取机制由两相反应转变为单相反应。当y = 0.05时,正极材料的放电容量增大,达到最大值120.2 mAh g-1;当y = 0.10时,放电容量减小。在C/3倍率和18°C条件下循环100次后,保留容量基本相同,其中y = 0.05, 94.0%为最佳。在50°C时,保留率下降了14%。该优化方案成功地应用于袋式电池50次循环,具有相似且稳定的电化学行为。因此,从Salar de Atacama获得的Mg(OH)2和Li2CO3都是Mg和Li的来源,是开发具有高倍率性能和长循环稳定性的锂离子电池正极材料的潜在候选材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


